Module 3
0.0(0)
Card Sorting
1/108
Earn XP
Description and Tags
Last updated 1:55 PM on 2/23/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
109 Terms
1
New cards
Define the term ionisation.
* The energy required to remove 1 mole of electrons from 1 mole of gaseous atoms to form 1 mole of gaseous +1 ions
2
New cards
Give the equation for the first ionisation of oxygen.
* O(g) → O+(G) + e-
3
New cards
Give the factors which affect ionisation energy.
* Nuclear charge → The more protons there are in the nucleus, the more positively charged the nucleus is and thus a stronger attraction for electrons
* Atomic radius → An electron close to the nucleus will be much more strongly attracted than one further away
* Shielding → As the number of electrons between the outer electrons and the nucleus increases, the outer electrons feel less attraction towards the nuclear charge. This lessening of the pull of the nucleus by inner shells of electrons is called shielding
* Atomic radius → An electron close to the nucleus will be much more strongly attracted than one further away
* Shielding → As the number of electrons between the outer electrons and the nucleus increases, the outer electrons feel less attraction towards the nuclear charge. This lessening of the pull of the nucleus by inner shells of electrons is called shielding
4
New cards
What is the trend in ionisation energy down a group?
* As you go down a group, ionisation energies tend to fall
* Elements further down a group have more electrons shells than the ones above. This means the atomic radius is larger and there is increased shielding making it easier to lose electrons.
* The increasing positive charge of the nucleus is overridden by the extra shells
* Elements further down a group have more electrons shells than the ones above. This means the atomic radius is larger and there is increased shielding making it easier to lose electrons.
* The increasing positive charge of the nucleus is overridden by the extra shells
5
New cards
What is the trend in ionisation energy across a period?
* As you move across a period, ionisation energies tend to increase
* This is because the number of protons in the nucleus is increasing, resulting in a higher nuclear charge, so a smaller atomic radius and a stronger attraction
* All the electrons are roughly at the same energy level and so even if the electrons are in different orbitals, there is generally little extra shielding
* This is because the number of protons in the nucleus is increasing, resulting in a higher nuclear charge, so a smaller atomic radius and a stronger attraction
* All the electrons are roughly at the same energy level and so even if the electrons are in different orbitals, there is generally little extra shielding
6
New cards
What are successive ionisation energies?
* Each time you remove an electron there is a successive ionisation energy
7
New cards
What is the drop in ionisation energies between group 2 and 3 due to?
* The outer electrons in group 3 are in a p-orbital, not an s-orbital
8
New cards
What is the drop in ionisation energies between group 5 and 6 due to?
* Electron repulsion
9
New cards
Give the equation for the second ionisation of oxygen.
* O+(g) → O2+(G) + e-
10
New cards
What are large jumps in ionisation energies due to?
* When a new shell is broken into, an electron from a shell closer to the nucleus is being removed
11
New cards
What is the trend in melting point across period 2 and 3?
* Generally, melting point increases for the first 4 elements, and then decrease for the final 4
* Metals melting point increases across a period due to increasing +ve charge, an increased number of delocalised electrons, and a decreasing ionic radius. This leads to a higher charge density, which attracts the ions together more strongly
* Elements with giant covalent structures (B, C and Si) have strong covalent bonds linking all their atoms together. A lot of energy is needed to break these bonds
* Simple molecular substances (N2, O2, F2, P4, S8 AND Cl2) also have covalent bonds between the atoms in a molecule. However, the melting points and boiling points depend upon the strength of the induced dipole-dipole forces between their molecules. These forces are weak and do not need a lot of energy to overcome, resulting in a low melting and boiling point. The more atoms in a molecule, the stronger the forces, so sulfur will have a higher melting point than phosphorus or chlorine
* The noble gases have the lowest melting and boiling points as they exist as individual atoms, resulting in very weak induced dipole-dipole forces
* Metals melting point increases across a period due to increasing +ve charge, an increased number of delocalised electrons, and a decreasing ionic radius. This leads to a higher charge density, which attracts the ions together more strongly
* Elements with giant covalent structures (B, C and Si) have strong covalent bonds linking all their atoms together. A lot of energy is needed to break these bonds
* Simple molecular substances (N2, O2, F2, P4, S8 AND Cl2) also have covalent bonds between the atoms in a molecule. However, the melting points and boiling points depend upon the strength of the induced dipole-dipole forces between their molecules. These forces are weak and do not need a lot of energy to overcome, resulting in a low melting and boiling point. The more atoms in a molecule, the stronger the forces, so sulfur will have a higher melting point than phosphorus or chlorine
* The noble gases have the lowest melting and boiling points as they exist as individual atoms, resulting in very weak induced dipole-dipole forces
12
New cards
What are the non ionisation trend followers?
* Al’s valence electron is in the 3p orbital which creates additional shielding. Therefore there is a slight dip in ionisation energy (also applies to S and P)
13
New cards
What is metallic bonding?
* Metal cations are electrostatically attracted to the delocalised electrons - they form a lattice of closely packed cations in a sea of delocalised electrons
14
New cards
How does metallic bonding affect melting and boiling point?
* The more delocalised electrons there are, the stronger the bonding will be and thus a higher melting and boiling point
* The size of the metal ion and the lattice structure will also affect it. A smaller ionic radius will hold the delocalised electrons closer to the nuclei
* The size of the metal ion and the lattice structure will also affect it. A smaller ionic radius will hold the delocalised electrons closer to the nuclei
15
New cards
Give the properties of metals.
* Malleable and ductile due to the fact that there are no real bonds holding specific ions together
* They can conduct electricity and heat
* They are insoluble, except in liquid metals, due to the strength of the metallic bonds
* They can conduct electricity and heat
* They are insoluble, except in liquid metals, due to the strength of the metallic bonds
16
New cards
Describe giant covalent lattices.
* Bonds between atoms are indefinite
* No discrete molecules
* Covalent bonding between all adjacent atoms
* Layers are held together by intermolecular forces with delocalised electrons between them
* No discrete molecules
* Covalent bonding between all adjacent atoms
* Layers are held together by intermolecular forces with delocalised electrons between them
17
New cards
What are the exceptions to the octet rule in regards to covalent bonding?
* Boron and beryllium don’t fulfil the octet rule but pair up unpaired electrons i.e. Boron trifluoride
18
New cards
Describe sulfur hexafluoride.
* Sulfur has 6 valence electrons
* 6 covalent bonds then form
* 12 electrons surround sulfur
* Fluorine achieves octet rule
* 6 covalent bonds then form
* 12 electrons surround sulfur
* Fluorine achieves octet rule
19
New cards
What is the structure of iodine?
* Large iodine molecules packed together causing a crystalline form of iodine. A molecular covalent crystal
* Brittle due to weak bonds
* Doesn’t conduct
* Low melting and boiling point
* Brittle due to weak bonds
* Doesn’t conduct
* Low melting and boiling point
20
New cards
Give the structure of diamond.
* Macromolecular structure
* High meting point
* Doesn’t conduct
* Tetrahedral
* High meting point
* Doesn’t conduct
* Tetrahedral
21
New cards
What are the trends for the group 2 alkaline earth metals?
* As you go down the group, ionisation energy decreases, so reactivity increases
* As you go down valence electrons become further away from the nucleus
* As you go down the group, more OH- ions are able to be released, so become more alkaline
* They can thermally decompose
* Used for neutralising acids
* Solubility increases down the group
* Reactivity increases down the group as atomic radius increases therefore a weaker nuclear attraction between the valence electrons
* Thermal stability increases down the group
* As you go down valence electrons become further away from the nucleus
* As you go down the group, more OH- ions are able to be released, so become more alkaline
* They can thermally decompose
* Used for neutralising acids
* Solubility increases down the group
* Reactivity increases down the group as atomic radius increases therefore a weaker nuclear attraction between the valence electrons
* Thermal stability increases down the group
22
New cards
Give a use of Ca(OH)2.
* Used in agriculture for neutralising acidic soil
23
New cards
Give a use of Mg(OH)2.
* Used to treat indigestion in antacids (as well as CaCO3)
24
New cards
How do you test for halide ions?
1) Add silver nitrate. A precipitate forms.
Cl- = White
Br- = Cream
I- = Yellow
2) Add NH3.
Cl- = Precip. dissolves in dilute NH3
Br- = Precip. dissolves in concentrated NH3
I- = Precip. is insoluble
Cl- = White
Br- = Cream
I- = Yellow
2) Add NH3.
Cl- = Precip. dissolves in dilute NH3
Br- = Precip. dissolves in concentrated NH3
I- = Precip. is insoluble
25
New cards
How do you test for halogens?
* Add X water with cyclohexane to KX
26
New cards
What are the colour changes you would expect to see when adding cyclohexane to test for halogens?
* Chlorine water and KBr → Yellow to orange
* Chlorine water and KI → Brown to violet
* Bromine water and KI → Brown to violet
* There would be no other visible changes as the halogens cannot be displaced in the other reactions
* Chlorine water and KI → Brown to violet
* Bromine water and KI → Brown to violet
* There would be no other visible changes as the halogens cannot be displaced in the other reactions
27
New cards
What are the trends in the group 7 halogens?
* As you go down the group reactivity decreases as atomic radius increases. Outer electrons are shielded more from the attraction of the nucleus due to more inner electrons. This makes it harder for large atoms to attract the electron needed to form an ion
* Boiling and melting point increase down the group due to the increasing strength of induced dipole-dipole forces. The number of electrons increases when the size and relative mass of the atoms increases
* Volatility decreases down the group
* They are oxidising agents
* Boiling and melting point increase down the group due to the increasing strength of induced dipole-dipole forces. The number of electrons increases when the size and relative mass of the atoms increases
* Volatility decreases down the group
* They are oxidising agents
28
New cards
How do you test for carbonate ions?
* Add an acid
* Positive result = fizzing
* Positive result = fizzing
29
New cards
What is the equation for testing for carbonate ions?
* H+(aq) + CO3-2(aq) → CO2(g) + H2O(l)
30
New cards
How do you test for halide ions?
* Add nitric acid
* Add silver nitrate and a precipitate will form
* AgCl = white precipitate
* AgBr = cream precipitate
* AgI = yellow precipitate
* Add silver nitrate and a precipitate will form
* AgCl = white precipitate
* AgBr = cream precipitate
* AgI = yellow precipitate
31
New cards
What is the equation for testing for halide ions?
* Ag+(aq) + X-(aq) → AgS(s)
32
New cards
How do you test for sulfate ions?
* Add barium chloride/nitrate
* A white precipitate will form
* A white precipitate will form
33
New cards
What is the equation for testing for sulfate ions?
* Ba2+(aq) + SO4-2(aq) → BaSO4(s)
34
New cards
How do you test for ammonium ions?
* Add aqueous sodium hydroxide
* Warm the mixture
* Test with damp red litmus paper
* A positive result will make it turn blue
* Warm the mixture
* Test with damp red litmus paper
* A positive result will make it turn blue
35
New cards
What is disproportionation?
* When a single element is simultaneously oxidised and reduced
36
New cards
When will a halogen undergo disproportionation? What is the equation?
* When they react with cold, dilute alkali solutions such as sodium hydroxide
* X2 + 2NaOH → NaXO + NaX + H2O
* X2 + 2NaOH → NaXO + NaX + H2O
37
New cards
What oxidation states can the halogens exist as (specific example?
* All of the halogens, except fluorine) can exist in oxidation states other than the 0 and -1 oxidation states
* Chloride ions (Cl-) → -1
* Chlorine (Cl2) → 0
* Chlorate (ClO-) → +1
* Chloride ions (Cl-) → -1
* Chlorine (Cl2) → 0
* Chlorate (ClO-) → +1
38
New cards
What happens when you mix chlorine with water? What is the equation for this?
* It undergoes disproportionation. You get a mixture of hydrochloric acid and chloric acid
* Cl2(g) + H2O(l) → HCl(aq) + HClO(aq)
* The above reaction is reversible
* Cl2(g) + H2O(l) → HCl(aq) + HClO(aq)
* The above reaction is reversible
39
New cards
What happens when you mix chlorine gas with cold, dilute aqueous sodium hydroxide? What is the equation for this?
* Sodium chlorate solution, NaClO(aq), is produced. (More commonly known as household bleach)
* 2NaOH(aq) + Cl2(g) → NaClO(aq) + NaCl(aq) + H2O(l)
* 2NaOH(aq) + Cl2(g) → NaClO(aq) + NaCl(aq) + H2O(l)
40
New cards
What type of a structure is a giant covalent lattice?
* A macromolecular structure
41
New cards
What is an allotrope?
* Different forms of the same element in the same state
42
New cards
Explain graphite’s structure and properties?
* The carbon atoms are arranged in flat sheets made up of hexagons
* The sheets are bonded together with weak induced dipole-dipole forces allowing layers to easily slide over each other
* Each carbon atom form 3 bonds. The fourth valence electron of each atom is delocalised which means an electric current can flow
* There are very strong covalent bonds between atoms in the sheets meaning graphite has a very high melting point
* Graphite is insoluble in any solvent as the covalent bonds are too strong to break
* The sheets are bonded together with weak induced dipole-dipole forces allowing layers to easily slide over each other
* Each carbon atom form 3 bonds. The fourth valence electron of each atom is delocalised which means an electric current can flow
* There are very strong covalent bonds between atoms in the sheets meaning graphite has a very high melting point
* Graphite is insoluble in any solvent as the covalent bonds are too strong to break
43
New cards
Explain diamond’s structure and properties.
* Each carbon atom is covalently bonded to four other carbon atoms
* The atoms are arranged tetrahedrally giving it its crystal lattice structure
* The strong covalent bonds give diamond a very high melting put
* It is very hard
* It cannot conduct
* It will not dissolve in any solvent
* The atoms are arranged tetrahedrally giving it its crystal lattice structure
* The strong covalent bonds give diamond a very high melting put
* It is very hard
* It cannot conduct
* It will not dissolve in any solvent
44
New cards
True or false?
Silicon has extremely different properties to diamond.
Silicon has extremely different properties to diamond.
* False
* Silicon has similar properties to diamond because it is in the same group as carbon and each atom can form four strong covalent bonds too. It will also form a crystal lattice structure, just like diamond does
* Silicon has similar properties to diamond because it is in the same group as carbon and each atom can form four strong covalent bonds too. It will also form a crystal lattice structure, just like diamond does
45
New cards
Explain the structure and properties of graphene.
* Graphene is a single layer of graphite
* It is a two-dimensional compound because it is only one atom thick
* The delocalised electrons are free to move along the sheet. Without layers they can move above and below the sheet, making graphene the best known electrical conductor
* Graphene is very strong due to the delocalised electrons which strengthen the covalent bonds
* It is transparent and extremely light
* It is a two-dimensional compound because it is only one atom thick
* The delocalised electrons are free to move along the sheet. Without layers they can move above and below the sheet, making graphene the best known electrical conductor
* Graphene is very strong due to the delocalised electrons which strengthen the covalent bonds
* It is transparent and extremely light
46
New cards
What is the reaction of a group 2 metal with:
a) water
b) oxygen
c) dilute acids
a) water
b) oxygen
c) dilute acids
a) M + 2H2O → M(OH)2 + H2
b) 2M + O2 → 2MO
c) M + 2HCl → MCl2 + H2
b) 2M + O2 → 2MO
c) M + 2HCl → MCl2 + H2
47
New cards
Why does adding chlorine to our water make it safe for us to drink?
* The chlorate ions kill bacteria
48
New cards
What are the alternatives to using chlorine to purify drinking water?
* Ozone → As a strong oxidising agent, it makes it easy t kill microorganisms. It is expensive to produce and has a short half-life meaning that its treatment is not permanent
* Ultraviolet light → Kills microorganisms by damaging their DNA. It is ineffective in cloudy water. Like O3, it won’t stop the water being contaminated down the line
* Ultraviolet light → Kills microorganisms by damaging their DNA. It is ineffective in cloudy water. Like O3, it won’t stop the water being contaminated down the line
49
New cards
What is enthalpy change?
* The heat energy transferred in a reaction at constant pressure in KJ mol-1
50
New cards
What are the standard conditions of enthalpy change?
* 100 kPa
* 298 K
* 298 K
51
New cards
What is the standard enthalpy change of reaction?
* The enthalpy change when a reaction occurs in the molar quantities shown in the chemical equation, under standard conditions with all the reactants and products in their standard conditions
52
New cards
What is standard enthalpy change of formation?
* The enthalpy change when 1 mole of a compound is formed from its elements in their standard states under standard conditions
53
New cards
What is standard enthalpy change of combustion?
* The enthalpy change when 1 mole of substance is completely burned in oxygen under standard conditions with all reactants and products in their standard states
54
New cards
What is the standard enthalpy change of neutralisation?
* The enthalpy change when solutions of an acid and alkali react together to form 1 mole of water under standard conditions
55
New cards
What is an exothermic reaction?
* Reactions that give out energy to their surroundings resulting in a negative enthalpy change
56
New cards
Draw an exothermic reaction profile?
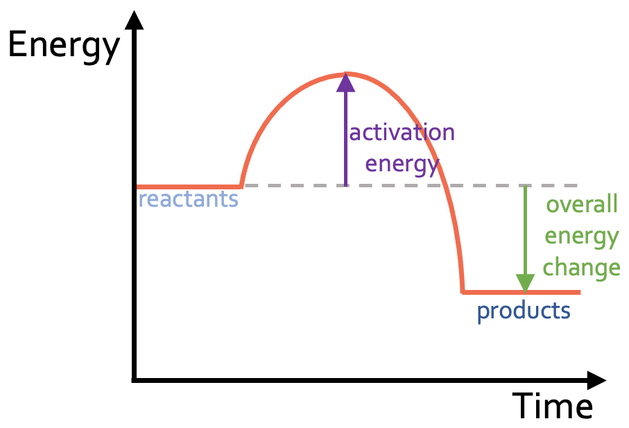
57
New cards
What is an endothermic reaction?
* Reactions that take energy from their surroundings resulting in a positive enthalpy change
58
New cards
Draw an endothermic reaction profile.
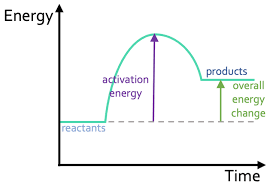
59
New cards
Define the term activation energy.
* The minimum amount of energy needed to begin breaking reactant bonds and start a chemical reaction
60
New cards
Define bond enthalpy.
* The energy required to break a bond between two atoms. Usually given as an ‘average bond enthalpy’
61
New cards
Is bond breaking exothermic or endothermic?
* You need energy to break the bonds so bond breaking is endothermic
62
New cards
Is bond making exothermic or endothermic?
* You release energy when making bonds so bond making is exothermic
63
New cards
What is average bond enthalpy?
* The energy needed to break one mole of bonds in the gas phase, averaged over many different compounds
64
New cards
How do you calculate the enthalpy change of reaction?
* Enthalpy change of reaction = Total energy absorbed - Total energy released (reactants-products)
65
New cards
What is the equation for enthalpy change?
* q = mcΔT
* m = mass (g) in the container, or mass of water
* c = specific heat capacity of the solution/water
* ΔT = temperature change of the solution/water (degrees Celsius or kelvin)
* q = heat lost or gained (J). This is the same as the enthalpy change if the pressure is constant
* m = mass (g) in the container, or mass of water
* c = specific heat capacity of the solution/water
* ΔT = temperature change of the solution/water (degrees Celsius or kelvin)
* q = heat lost or gained (J). This is the same as the enthalpy change if the pressure is constant
66
New cards
What is Hess’ Law?
* The total enthalpy change of a reaction is always the same, no matter which route is taken
67
New cards
What is the value of enthalpy change of formation for elements?
* If they are in their elemental state, the enthalpy change of formation will be zero
68
New cards
What does collision theory state?
* A reaction will not take place between two particles unless they collide in the right direction (they need to be facing each other the right way) and they collide with at least a certain minimum amount of kinetic energy
69
New cards
What is the Boltzmann distribution curve?
* A graph that shows the distribution of energies at a certain temperature
70
New cards
Draw the Boltzmann distribution curve with explanations for each point.
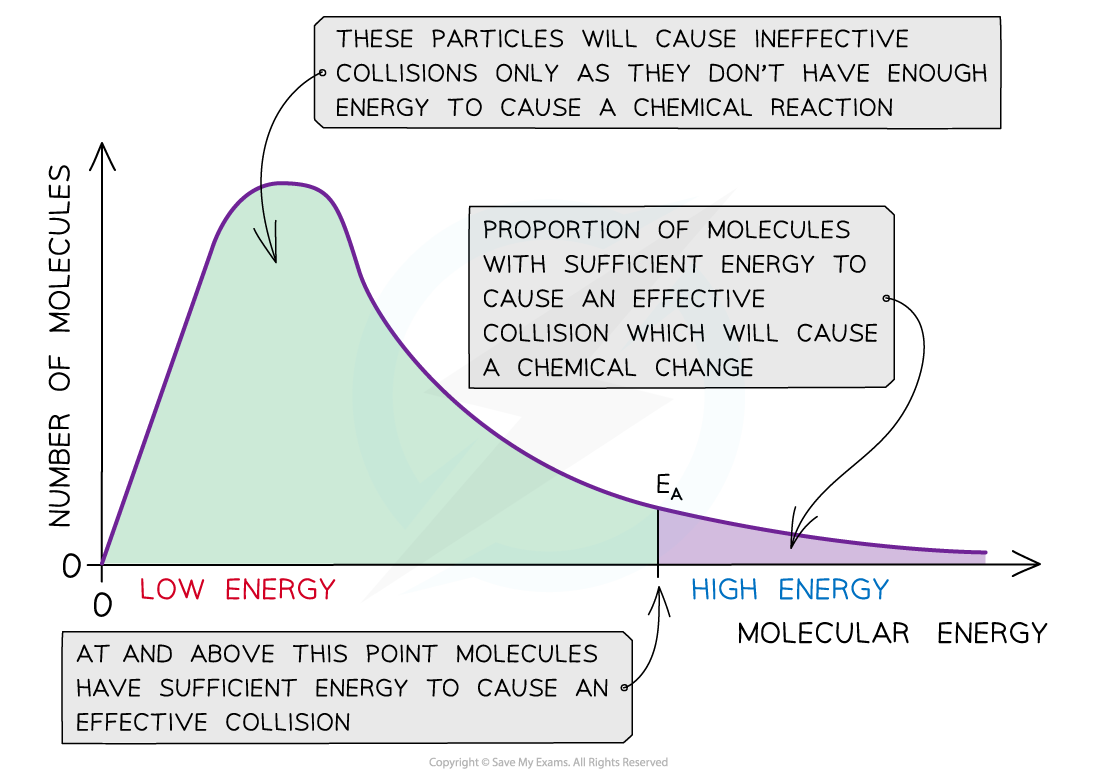
71
New cards
Why does the Boltz. curve have to go through the origin/
* It must go through the origin as there are no molecules with no energy
72
New cards
True or false?
The energy distribution in a Boltz. curve should never meet the x-axis.
The energy distribution in a Boltz. curve should never meet the x-axis.
* True. The curve should never meet the x-axis as there is no maximum energy for molecules
73
New cards
What does the area under the Boltz. curve represent?
* The total number of particles present
74
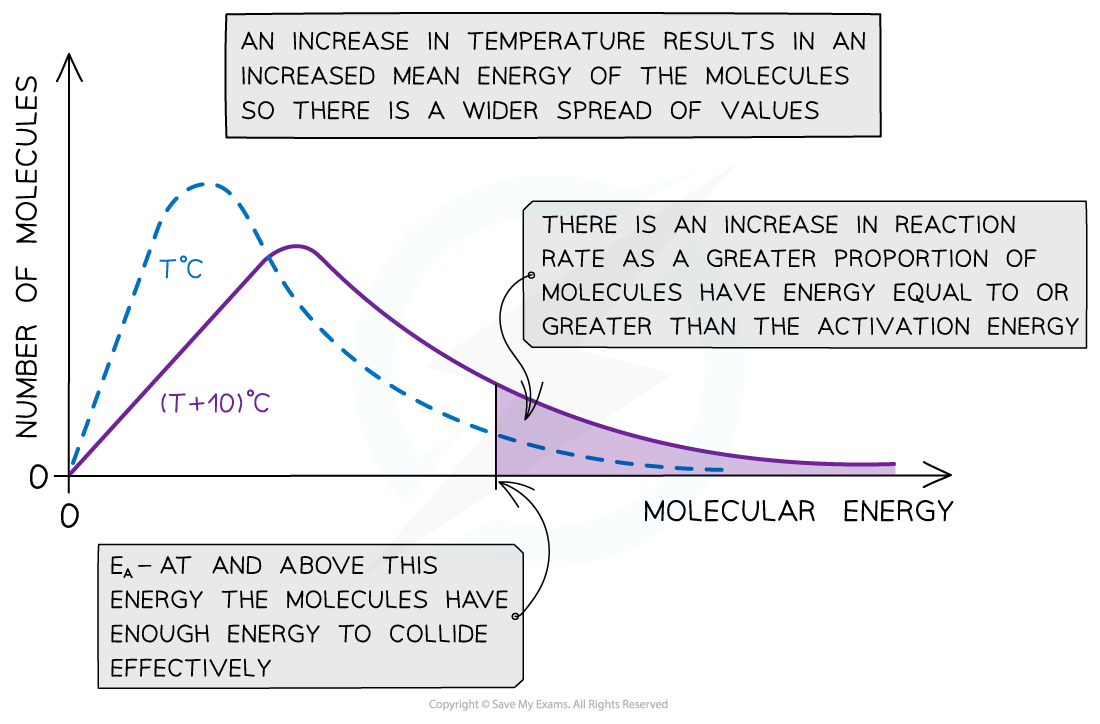
New cards
How will temperature effect the rate of reaction and the shape of the Boltzmann distribution curve?
* If you increase the temperature of a substance, the molecules will, on average, have more kinetic energy and thus move faster
* This means a greater proportion of molecules will have at least the activation energy and be able to react
* This will slightly flatten, and push the Boltzmann distribution curve over to the right. The number of molecules is still the same, so the area under the curve must not change
* This means a greater proportion of molecules will have at least the activation energy and be able to react
* This will slightly flatten, and push the Boltzmann distribution curve over to the right. The number of molecules is still the same, so the area under the curve must not change
75
New cards
How will concentration effect the rate of reaction and the shape of the Boltzmann distribution curve?
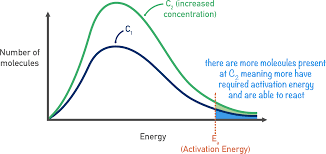
* By increasing concentration of reactants, the particles will be closer together, meaning more frequent collision. This increases the chances of them reacting
* The activation energy won’t change but the number of particles that can achieve that minimum energy will increase
\
* The activation energy won’t change but the number of particles that can achieve that minimum energy will increase
\
76
New cards
True or false?
If the reactants are solids, increasing the pressure will increase the rate of reaction?
If the reactants are solids, increasing the pressure will increase the rate of reaction?
* False
* If the reactants are gases, then increasing the pressure will increase the rate of reaction
* If the reactants are gases, then increasing the pressure will increase the rate of reaction
77
New cards
What is a catalyst?
* A catalyst increases the rate of reaction by providing an alternative reaction pathway with a lower activation energy
* The catalyst will be chemically unchanged by the end of the reaction
* The catalyst will be chemically unchanged by the end of the reaction
78
New cards
How do catalysts work?
* The reactant molecules bind to the catalyst making it easier to break the bonds, reducing the activation energy
* The broken reactant molecules then form product molecules, and break away from the catalyst
* The broken reactant molecules then form product molecules, and break away from the catalyst
79
New cards
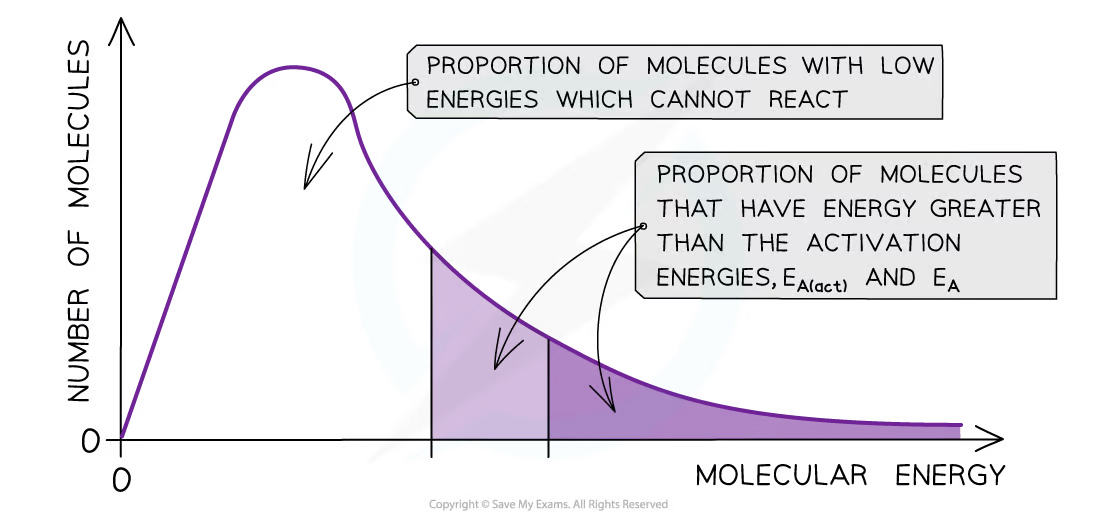
How will the addition of a catalyst change the rate of reaction and the Boltzmann distribution curve?
* By lowering the activation energy, a greater proportion of molecules in the reaction mixture have the activation energy, and therefore have sufficient energy for an effective collision
* The rate of the catalysed reaction is increased compared to the uncatalyzed reaction
* The rate of the catalysed reaction is increased compared to the uncatalyzed reaction
80
New cards
What is a heterogenous catalyst?
* A catalyst that is in a different phase from the reactants
81
New cards
What is a homogenous catalyst?
* A catalyst which is in the same physical state as the reactants
* Usually an aqueous catalyst
* Usually an aqueous catalyst
82
New cards
How does a heterogenous catalyst work?
* The reaction happens on the surface of the catalyst so increasing surface area of the catalyst will increase rate of reaction
83
New cards
How does a homogenous catalyst work?
* It forms an intermediate species. One or more reactant combine with the catalyst to make the intermediate species, which then reacts to form the products and reform the catalyst
84
New cards
Why are catalysts used in industry?
* They dramatically lower production costs, and help make products better
* I.e. Polyethene is less dense and less rigid when made without a catalyst. The use of a catalyst also increases its melting point
* I.e. Polyethene is less dense and less rigid when made without a catalyst. The use of a catalyst also increases its melting point
85
New cards
How do catalytic converters in cars reduce the pollution released into the atmosphere?
* They speed up the following reaction:
2CO + 2NO → 2CO2 + N2
2CO + 2NO → 2CO2 + N2
86
New cards
Do catalysts last forever?
* No
* Eventually they need to be disposed of. However, many contain valuable and/or toxic compounds. This means they must either be recycled, or disposed of by special companies
* Eventually they need to be disposed of. However, many contain valuable and/or toxic compounds. This means they must either be recycled, or disposed of by special companies
87
New cards
Define reaction rates.
* How fast a reaction takes place
* The change in the amount of reactant or products per unit time
* The change in the amount of reactant or products per unit time
88
New cards
What is the equation for calculating rate of reaction?
RoR = Amount of reactant used or product formed / Time
89
New cards
What properties can you choose to follow in a reaction to determine the rate of reaction
* Change in mass
* Gas volume
* Amount of reactant used
* Amount of product formed
* Changes in pressure (gases only)
* Changes in colour (solutions only)
* Changes in conductivity
* Gas volume
* Amount of reactant used
* Amount of product formed
* Changes in pressure (gases only)
* Changes in colour (solutions only)
* Changes in conductivity
90
New cards
How can you calculate rate of reaction from a graph?
* The gradient
* If the graph is a curve, then you need to use a tangent
* If the graph is a curve, then you need to use a tangent
91
New cards
True or false?
As the reactants get used up, the forward reaction slows down - and as more product is formed, the reverse reaction speeds up.
As the reactants get used up, the forward reaction slows down - and as more product is formed, the reverse reaction speeds up.
True
92
New cards
When, in a reversible reaction, will it appear that nothings is happening?
* When the forwards and backwards reactions are happening at the same rate because the concentration of reactants and products won’t be changing anymore
93
New cards
What is dynamic equilibrium?
* When the rate of reaction for the forwards and backwards reactions are equal
* It can only happen in a closed system
* It can only happen in a closed system
94
New cards
What is Le Chatelier’s principle?
* If there is a change in concentration, pressure or temperature, the equilibrium will move to help counteract the change
95
New cards
Will the use of a catalyst change the position of equilibrium?
* No, the use of a catalyst will have no affect on the position of equilibrium
96
New cards
How does increasing the concentration of reactants affect equilibrium?
* If you increase the conc. of reactants, equilibrium will try to get rid of them by making more product, meaning equilibrium has shifted to the right
* If you were to increase the conc. of the products, equilibrium would combat this by increasing the speed of the reverse reaction
* Decreasing the conc. has the opposite effect
* If you were to increase the conc. of the products, equilibrium would combat this by increasing the speed of the reverse reaction
* Decreasing the conc. has the opposite effect
97
New cards
When will changing the pressure affect equilibria?
* If there are gases involved
98
New cards
How does increasing the pressure shift equilibrium?
* Increasing the pressure shifts equilibrium to favour the side with the fewest gas molecules, reducing the pressure
* Decreasing pressure will favour the side with the most gas molecules, raising the pressure
* Decreasing pressure will favour the side with the most gas molecules, raising the pressure
99
New cards
How will changing the pressure affect the position of equilibria?
* Increasing the pressure will make equilibrium shift to favour the endothermic reaction in order to absorb the energy
* Decreasing the temperature will mean equilibrium shifts to favour the exothermic reaction in order to replace the heat energy
* Decreasing the temperature will mean equilibrium shifts to favour the exothermic reaction in order to replace the heat energy
100
New cards
How is ethanol made?
* By reacting ethene with steam
* The industrial conditions are: a pressure of 60-70 atmospheres, a temperature of 300 degrees Celsius and a phosphoric acid catalyst
* The reaction is reversibleW
* The industrial conditions are: a pressure of 60-70 atmospheres, a temperature of 300 degrees Celsius and a phosphoric acid catalyst
* The reaction is reversibleW