enviro final
0.0(0)
Card Sorting
1/211
Earn XP
Description and Tags
Last updated 10:45 PM on 12/20/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
212 Terms
1
New cards
1. **What are the four primary units in this course?**
Energy, Carbon, Food, and Waste
2
New cards
What three things do definitions of sustainability tend to focus on?
(1) meeting human needs,
(2) conserving resources, and
(3) intergenerational equity.
(2) conserving resources, and
(3) intergenerational equity.
3
New cards
1. **Sustainability means different things to different people depending on the scale or context. What would be** ***your*** **definition of sustainability as you begin this semester?**
**Sustainability is practicing and maintaining planet-conscious and energy-conscious habits within my own individual experience.**
4
New cards
What are the three “bottom lines” of sustainability, sometimes referred to as the “three P’s”?
People, Planet, Profit (or society, economy, environment)
5
New cards
Lecture 2A presented two models of the triple bottom line: a Venn diagram model and a nested model. Pick one of these models and explain how the model depicts sustainability.
Venn diagram: Sustainable actions or processes are those that bring positive returns to the economy, the environment, and society
Nested: A healthy economy depends upon a healthy and functioning society, and a healthy society depends upon a healthy and functioning environment.
\
Nested: A healthy economy depends upon a healthy and functioning society, and a healthy society depends upon a healthy and functioning environment.
\
6
New cards
Lecture 2A also introduced the term “carbon footprint” and noted that this term includes both a shorthand and a metaphor. **Which is the shorthand and what is it short for?** Which is the metaphor and what is it a metaphor for?
**CO2e - carbon dioxide equivalent \\ “Footprint” is a metaphor for the impact that the carbon dioxide proposed has on the environment**
7
New cards
1. **Why do your instructors use purple clouds to represent carbon dioxide emissions?**
CO2 is invisible to humans (colorless, odorless, tasteless) so purple clouds help to visualize carbon dioxide.
8
New cards
1. **For a kW-h:**
1. **What do those letters stand for and what do they measure?**
**kilowatt-hour; units of electricity**
9
New cards
What is a connection between this unit of measurement and sustainability?
kW-h measures electricity, and our current system for supplying electricity is not sustainable. For example, much electricity generation relies on burning fossil fuels that are not renewable and contribute to climate change.
10
New cards
1. **Here is the graph that was introduced in the lecture. Note that it is missing its labels.**
What is being measured on the y-axis (vertical axis) for the brown line? Include the name ***and*** the units.
**Gross square feet are being measured in this graph depicting energy and buildings**
\n
\n
11
New cards
Provide the name and units for what is measured on the y-axis (vertical axis) for the blue line of the graph shown in lecture.
Name: campus energy use (or something similar). Units: million British thermal units (or MMBTU).
12
New cards
In Wisconsin, something notable happened in 2019 regarding electricity generation. What was it?
Coal-fired power plants provided less than half (42%) of statewide electricity generation for the first time in over three decades.
13
New cards
The discussion listed five projects from FP&M (campus facilities) that caused the decrease in campus energy use. Name three of them.
**changes in lab ventilation/frequency of air exchanges in classrooms/labs, lighting upgrades, new behaviors by building occupants**
14
New cards
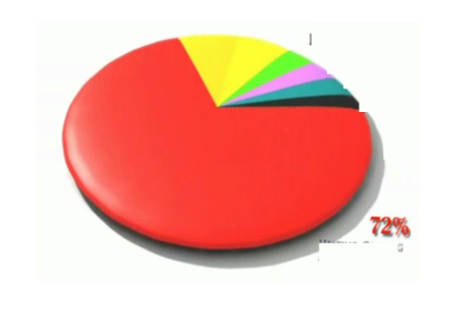
This pie chart showing campus energy use is missing its labels. What is the biggest section of the pie chart (72%), shown in red?
HVAC
15
New cards
Check the items that are TRUE for carbon dioxide
As a gas, carbon dioxide is not detectable by humans
Carbon dioxide is more than 1% of our atmosphere
Carbon dioxide is a greenhouse gas
Carbon dioxide is able to burn
Carbon dioxide is produced when fossil fuels
The air that you exhale contains carbon dioxide
The air that you inhale contains carbon dioxide
\
As a gas, carbon dioxide is not detectable by humans
Carbon dioxide is more than 1% of our atmosphere
Carbon dioxide is a greenhouse gas
Carbon dioxide is able to burn
Carbon dioxide is produced when fossil fuels
The air that you exhale contains carbon dioxide
The air that you inhale contains carbon dioxide
\
T
F
T
F
T
T
Most likely, but only a tiny bit
F
T
F
T
T
Most likely, but only a tiny bit
16
New cards
Part a: What does the “e” stand for?
Part a: e = equivalence (or equivalent)
17
New cards
What does the unit CO2e describe (i.e. how is it different than just CO2)?
CO2e describes the climate change impact of all greenhouse gasses expressed as an equivalent mass of carbon dioxide (compared to just CO2).
18
New cards
Apply systems thinking to an example from your own life. You can describe an effect in either time or space, but you don’t need to do both.
**I can think about using a bus to get to a class. An effect in space is that the emissions from this bus will enter the atmosphere and permeate throughout the greater ecosystem on earth.**
19
New cards
**__** __**describes a** ***rate of change*****, and**__ **__ describes a** ***total sum*** **after accounting for a length of time. Which is which?**
Power describes a rate of change, and energy describes a total sum after accounting for a length of time.
20
New cards
1. **What is the standard unit for power? Provide the name** ***and*** **abbreviation.**
**Watts (W)**
\n
\n
21
New cards
Provide three examples of units of measurement for energy.
Joule, calorie, therm
22
New cards
What is the relationship between a joule and a watt-second?
A joule and a watt-second are equivalent. They describe an equal amount of energy.
23
New cards

Identify the error in the lighting facts label.
The label lists “Energy Used” and then provides a value in watts (power, not energy).
24
New cards
Explain the relationship between power and energy, as if you were teaching a 6th grader. Provide an example or an analogy if that helps your explanation.
**E = p*t – this relationship is a simple one. Think of it like speed (mi/hr) vs distance! One of the characteristics (power) is an element used to calculate the other (energy)! Energy a rate of power/time.**
25
New cards

This photo shows a reaction fueled by chemical energy. Name the two forms of energy to which the chemical energy is being converted.
The chemical energy is converted into light and heat.
26
New cards
\
How many watt-seconds are there in one kilowatt-hour? Follow the process shown in the lecture slides, using the fact that there are 1,000 watts in a kilowatt and 3,600 seconds in an hour. Make sure your units cancel.
How many watt-seconds are there in one kilowatt-hour? Follow the process shown in the lecture slides, using the fact that there are 1,000 watts in a kilowatt and 3,600 seconds in an hour. Make sure your units cancel.
1 kW 1000 W 1 hr
—--------- X —---------- X —----------- = 0.28 W-S
1 hr 1 kW 3600 sec
—--------- X —---------- X —----------- = 0.28 W-S
1 hr 1 kW 3600 sec
27
New cards
A slide from lecture 3B featured a quote from Bananas about the carbon footprint of drying hands versus transatlantic flights. The title of the slide is “Perspective.” What message about having perspective and carbon footprints is the quote trying to communicate?
**The quote is trying to communicate that when considering sustainability, people should try to maintain** ***perspective*****. By that, they mean to remind yourself of the scale of the impact of your actions as it relates to your greater carbon footprint. You can feel guilty about every little thing that leaves a carbon footprint, but it is important to maintain the idea that you have to scale your actions while acting sustainability.**
\n
\n
28
New cards
1. **The discussion about electric power mentioned six fuel sources used to generate electricity. Name two that emit CO2 and two that are carbon-free (for generation only).**
**burning a fuel and transforming chemical to mechanical to thermal energy both emit carbon dioxide and spinning a turbine and making steam do not emit carbon dioxide.**
29
New cards
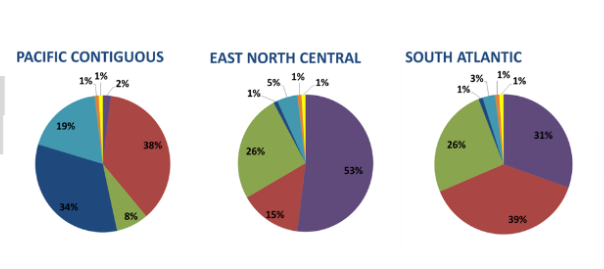
Here is an image from lecture. Explain what the pie charts represent *and* use the information to rank the three regions from lowest to highest carbon footprint per kilowatt-hour of electricity.
1. **The East North Central**
2. **South Atlantic**
3. **Pacific Contiguous**
30
New cards
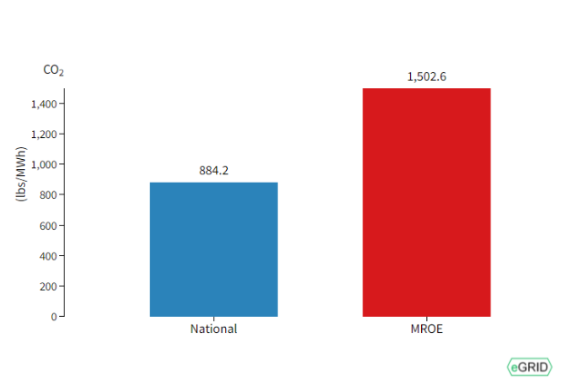
1. **Here is an image from lecture (2021 data).**
1. **What are the bars measuring?**
pounds of CO2e/MW-h
31
New cards
Why is the value (CO2e/kWh) for Wisconsin (MROE) higher than the national average?
The MROE value is higher than the national average because of reliance on coal.
32
New cards
Why are the values lower than in 2019?
Both nationally and in WI, coal is gradually being phased out in favor of less carbon-intensive fuel sources (like natural gas or wind/solar).
33
New cards
Electricity has a carbon footprint. When the WID uses electricity, where do the CO2 emissions come from?
1 point for general explanation that CO2 is emitted at the locations where the energy/electricity is generated. Full credit if a local example for the WID/UW-Madison also is given, such as MG&E power stations (Columbia, Blount, Cogen, etc.).
34
New cards
Suggest three reasons for why it’s important to ventilate air inside buildings.
which can include removing harmful fumes, removing waste heat from people or machines, expelling excess carbon dioxide, bringing in fresh oxygen, etc.
35
New cards
Describe two TBL-related considerations that connect to the decision to increase the ventilation frequency inside campus buildings.
1 point each for making a reasonable TBL connection. For example, the decision was made to protect the health of the campus community, as more frequent ventilation can mitigate the spread of coronavirus indoors. However, this results in additional energy use from HVAC which has both economic (cost of fuel) and environmental/social (CO2e and air quality) considerations.
36
New cards
Explain how an incandescent light bulb produces light and why this process is so inefficient.
Incandescent bulbs heat a tungsten filament to the point that it glows white hot. It’s essentially a heater that produces some light. 90% of the energy goes to heating the filament, 10% to light.
37
New cards
The filaments of incandescent light bulbs are sealed inside of glass.
Name the inert (noble) gas used to fill the bulbs.
To the nearest whole number, what % is the abundance of this gas in the atmosphere?
Why is this gas necessary inside incandescent bulbs?
Name the inert (noble) gas used to fill the bulbs.
To the nearest whole number, what % is the abundance of this gas in the atmosphere?
Why is this gas necessary inside incandescent bulbs?
Part a: the gas is argon. Part b: argon is roughly 1% of the atmosphere. Part c: argon is a noble gas and is inert, so it prevents the filament inside the incandescent bulb from combusting.
38
New cards
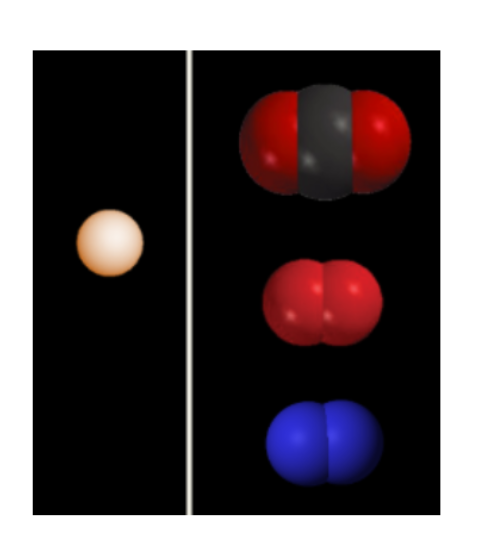
1. **The image to the right is from lecture 4A:**
1. **Identify each atmospheric gas by its** ***chemical name*** **and its** ***chemical formula*****. For example, the chemical formula for water is H2O, and the chemical name is, well…..water (it’s only dihydrogen monoxide for extra eyerolls).**
**carbon dioxide - CO2**
**(di)oxygen (oxygen gas naturally occurring) - O2**
**nitrogen gas - N22**
**argon (gas) - Ar**
**(di)oxygen (oxygen gas naturally occurring) - O2**
**nitrogen gas - N22**
**argon (gas) - Ar**
39
New cards
Rank the same four gasses by their abundance in air, from highest to lowest. You don’t need to recall specific percentages, only the relative order.
carbon dioxide (lowest) → argon → oxygen → nitrogen (highest)
40
New cards
1. **Fill in the blanks using information from lecture slides.**
1. Substances burn by reacting with **___** in the air. The product of combustion is an oxide. If the substance burned contained carbon, a product is *. If the substance burned contained hydrogen, a product is* __. Metals such as ()*or ()* burn to produce a metal oxide.
\n
oxygen, carbon dioxide, water, iron, magnesium
41
New cards
Write the chemical equation for the combustion reaction that takes place at the Charter Street plant. You can use either chemical names or chemical formulas, but you do *not* need to balance the equation.
methane (CH4) + oxygen (O2) = carbon dioxide (CO2) + water vapor (H2O)
42
New cards

Answer the question on the slide and explain your rationale.
Yes. The Columbia Energy Center burns coal as its primary fuel, and coal contains mercury. When coal burns, mercury also combusts and is emitted into the atmosphere.
43
New cards
Describe how a fluorescent bulb produces light (hint: zap….glow….glow).
ZAP: an electric discharge excites mercury atoms inside the fluorescent tube. GLOW: the mercury atoms emit ultraviolet light. GLOW: the phosphor coating inside the tube converts the UV light to visible light.
44
New cards

Answer the question on this slide from lecture. Explain your reasoning, assuming the electricity is provided in Wisconsin.
One point each for the correct answer with explanation. Since Wisconsin’s electricity is still primarily coal-fired, an incandescent bulb ultimately will be responsible for more mercury in the environment due to coal combustion.
45
New cards
1. **Campus heating and cooling.**
1. **What are three things that our campus power plants all have in common?**
**linkage by steam tunnels, emission of carbon dioxide/air pollutants, purifying lake water**
46
New cards
Name three substances that are monitored by the folks at Charter Street.
Carbon dioxide (CO2), carbon monoxide (CO), oxides of nitrogen (NOx aka NO or NO2).
47
New cards
Of the five campus energy projects (Lecture 2B), list two that impact campus HVAC.
Options include: coal to natural gas as the primary fuel, steam tunnel insulation, and ventilation optimization.
48
New cards
1. **For the equation E=mcΔT:**
Provide the name *and* units used for E, m, c, and ΔT.
1. **E means heat (or energy) and is measured in Joules**
2. **m means mass and is measured in grams**
3. **c means specific heat and is measured in Joules/grams-degrees Celsius**
1. **ΔT means change in temperature and is measured in degrees Celsius**
49
New cards
Using your answer from part a, substitute these units into the equation E=mcΔT. Show that the equation is balanced on both sides by canceling out the units.
see image
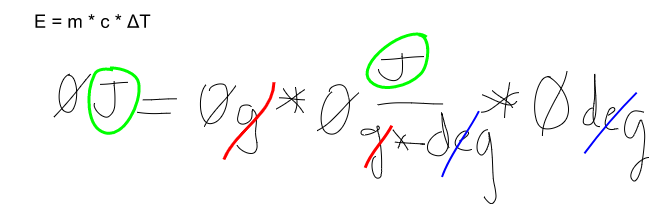
50
New cards
Consider the life cycle of the water you might use when taking a shower. Name two additional instances of energy use related to the water aside from heating.
Examples include energy to pump the water to you and energy to treat the water at a sewage plant after it goes down the drain.
51
New cards
Which two sectors in the United States account for nearly 80% of national groundwater use?
Thermoelectric (power) generation and agriculture.
52
New cards
How much energy in joules is required to heat 1.33 liters of water in the Meiko K-200? The water is heated from 30 °C to 72 °C.
**E = mc delta T**
**1.33 L * (1000 mL/1 L) = 1330 mL = 1330 g * 4.19 * 42 = 234, 053.4 J**
**1.33 L * (1000 mL/1 L) = 1330 mL = 1330 g * 4.19 * 42 = 234, 053.4 J**
53
New cards
1. **A joule is tiny compared to a kilowatt-hour: there are 3.6 million joules in one kW-h. How many kilowatt-hours does it take to heat this volume of water?**
**234, 053.4 J * (3,600,000 J / 1 kW-h) = 8.43e11 kW-h**
54
New cards
1. **What is the economic and planetary cost of this energy use? Use $0.11 per kilowatt-hour and 660 g CO2e per kilowatt-hour as conversion factors.**
**8.43e11 kW-h * $0.11/kW-h = 92,685,146,400**
**8.43e11 kW-h * 660 g CO2e/kW-h = 5.56e14 g CO2e**
\n
**8.43e11 kW-h * 660 g CO2e/kW-h = 5.56e14 g CO2e**
\n
55
New cards

1. **Here is a screen shot from Bananas.**
1. **The flames shouldn’t go up around the sides of a saucepan or a kettle. Explain using the logic presented by Mike Berners-Lee.**
**The flames being released will end in a net loss of energy as the open flame will lose energy as the heat from the flame enters the air in the surrounding room instead of contributing to the heating of the water.**
\n
\n
56
New cards
A saucepan should have the lid on when used to heat water. Explain using the logic presented by Mike Berners-Lee.
This improves heating efficiency by preventing heat loss to the surrounding air.
57
New cards
1. **The Charter Street Heating & Cooling Plant (CSHP).**
The primary fuel at CSHP is natural gas. Provide the chemical name *and* formula for **natural gas.**
methane - nh4
58
New cards
1. **What are** ***two*** **ways that CSHP uses the high pressure steam generated at the plant?**
HPS is used to heat the campus and also to drive motors that power the chiller units at CSHP. It's also okay if electricity generation is mentioned, as CSHP does generate a little electricity.
59
New cards
The combustion of natural gas is hot enough to react N2 and O2 and produce nitrogen monoxide (NO), an air pollutant. Name three natural sources of this pollutant.
volcanoes, forest fires, and lightning
60
New cards
Why is HPS generated centrally at CSHP rather than at each campus building? (Hint: the *Journey into the Madison Underground* video from lecture 4B has this answer)
**It’ll be more efficient than separating the bioliers between multiple buildings across campus.**
61
New cards
1. **Water at CSHP.**
1. **Water from Lake Mendota is purified in a water treatment building before being transformed into high pressure steam. Why must this water be so very, very pure?**
**to avoid mineral deposits in the boiler and steam pipe networks and also to avoid microorganisms and other contaminants**
62
New cards
What is condensate?
Condensate is the (hot) water that returns to CSHP after giving up its heat to campus buildings.
63
New cards
Provide two reasons why workers at CSHP try to conserve and reuse condensate.
Condensate is already purified and it's also hot water, so it makes energy and economic sense to conserve and reuse the condensate. And since less energy is being used, this also means less air pollutants and greenhouse gases emitted by the plant. Any two reasons that connect to these points are acceptable.
64
New cards
List *three* properties of refrigerant gasses.
colorless, tasteless, odorless
65
New cards
1. **The photos to the right connect to a recent efficiency upgrade at CSHP. Explain how these tiny foam balls benefit each component of the triple bottom line: the environment, the economy, and society.**
Environment: this project reduces the natural gas burned at the plant and GHGs released.
\
Economy: this saves the plant over $200,000/yea
\
Society: reduced natural gas combustion also leads to reduction of air pollutants emitted out the stacks, benefiting air quality and human health. \n
\
Economy: this saves the plant over $200,000/yea
\
Society: reduced natural gas combustion also leads to reduction of air pollutants emitted out the stacks, benefiting air quality and human health. \n
66
New cards
1. **A slide in lecture compared what happens at the CSHP cooling towers to what happens when we sweat on a hot day. How are these processes similar?**
**We expel some other kind of energy (sweat) like the plant does that cools the body by exposure to a colder external environment**
67
New cards
What does the term “cogeneration” mean? Provide a scenario of cogeneration from a power plant on our campus.
One point for the explanation and one point for the example. Cogeneration refers to the simultaneous production of multiple utilities from a power plant. Any example of CSHP or the Cogen plant producing multiple utilities at once is acceptable.
68
New cards
What is fuel oil, and why is it necessary to keep a 900,000 gallon tank of it at CSHP?
**a mixture of hydrocarbons derived from petroleum. it’s necessary because it serves as a backup if ever the university were to run out of methane**
69
New cards
Explain the term *hydrocarbon* and provide an example of a substance that is a hydrocarbon.
**a compound of ONLY hydrogen and carbon in any combination.an example is methane!**
70
New cards
1. **Prior to burning natural gas, CSHP burned coal as its fuel source. List** ***two*** **substances contained in coal that produce air pollutants when burned. For one of those substances, provide a chemical formula for the combustion reaction.**
Full credit for listing 2 impurities (no chemical equation needed for full credit). Options we've discussed are mercury and sulfur (though coal has more impurities like arsenic, radioactive substances, etc).
**CO2 and NO**
**N2 + O2 → 2NO**
**CO2 and NO**
**N2 + O2 → 2NO**
71
New cards
Indicate which statements below are true for NOx, N2O, or both. (first three statements only)
Air pollutant harmful to human health:
Associated with Agricultural emissions:
Potent greenhouse gas:
Emitted from stacks at Charter Street:
Can have human and non-human sources:
Can be used in anesthesia applications:
Air pollutant harmful to human health:
Associated with Agricultural emissions:
Potent greenhouse gas:
Emitted from stacks at Charter Street:
Can have human and non-human sources:
Can be used in anesthesia applications:
NOx
N2O
N2O
NOx
both
N2O
N2O
N2O
NOx
both
N2O
72
New cards
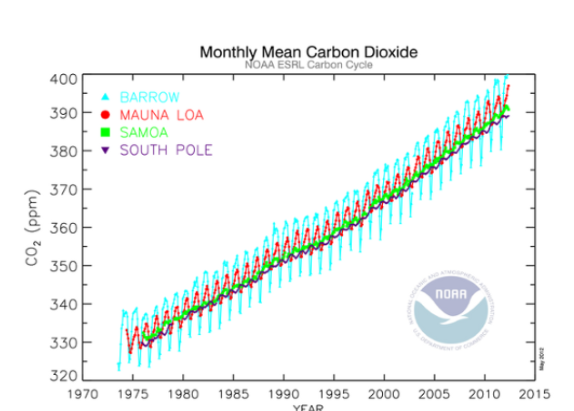
What accounts for the sawtooth pattern seen in the graph?
The seasonal cycles of photosynthetic plant life on the planet (particularly north of the equator).
73
New cards
What accounts for the *variation in the sawtooth* between the four stations?
The extremes that each of the stations will take on are very different because the location of each is very different. The northern hemisphere is more sensitive to annual CO2 variation. This is caused in part by the local plant life and wind patterns.
74
New cards
Explain what is happening to the oceans as they absorb more carbon and describe one effect of this on marine ecosystems.
The oceans are becoming more acidic with higher amounts of dissolved CO2. Effects could be on coral reefs (bleaching), organisms that make shells, or other reasonable suggestions.
75
New cards
Describe the “double hit” of deforestation to the carbon cycle mentioned in lecture.
Deforestation results in direct emissions of CO2 to the atmosphere from the burning of fuels for heavy machinery and the fires that are often accompanied by deforestation. Moreover, a reduction in plantlife limits photosynthetic activity that removes CO2 from the atmosphere.
76
New cards
1. **Describe a connection between energy use, population, and atmospheric CO2 levels.**
If populations use energy in respiration (to “burn food”), carbon dioxide will by a byproduct
77
New cards
Arrange these words in a chemical equation to represent photosynthesis.
water, energy (sunlight), oxygen, carbon dioxide, oxygen
water, energy (sunlight), oxygen, carbon dioxide, oxygen
carbon dioxide + water + energy (sunlight) → glucose + oxygen
\
1. glucose + oxygen → carbon dioxide + water + energy
1. respiration
\
1. glucose + oxygen → carbon dioxide + water + energy
1. respiration
78
New cards
If plants perform both respiration *and* photosynthesis, why are they considered carbon sinks (i.e. have a negative carbon balance)?
Plants inhale (photosynthesize) more CO2 than they exhale (respire) leaving negative carbon balance because carbon dioxide is a *reactant* in photosynthesis
79
New cards
Before fossil fuels, what is the original source of energy from which all living plants (and other photosynthetic organisms) use to grow?
Visible light (to use in photosynthesis)
80
New cards
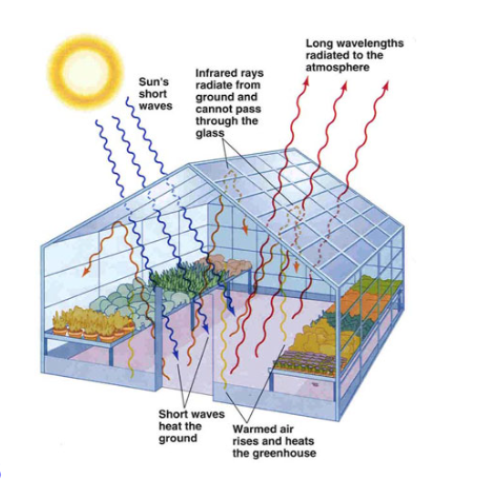
The image to the right is from lecture 7B. Describe how the atmosphere of our planet resembles a greenhouse.
Like a greenhouse, our atmosphere lets in solar energy but then traps the thermal energy emitted by the surface of the planet after it is heated by solar energy.
81
New cards
Explain what happens when a GHG molecule interacts with the right levels of IR energy (hint: ABB Warm).
The GHG molecule (1) absorbs the IR energy, it (2) bends or stretches its bonds, and it (3) bumps into other molecules in the atmosphere, causing them to move faster
82
New cards
1. **Now connect your answer to part a with an effect on atmospheric temperatures (make sure to reference N2 and O2).**
If GHGs latch on to IR energy levels, other atmospheric gasses like N2 and O2 will suffer alongside the rest of atmospheric gasses. This could run the risk of oxidation and combustion for these gasses more often if the thermal energy latched to GHGs raises the atmospheric temperature. This could cause a multitude of atmospheric problems.
83
New cards
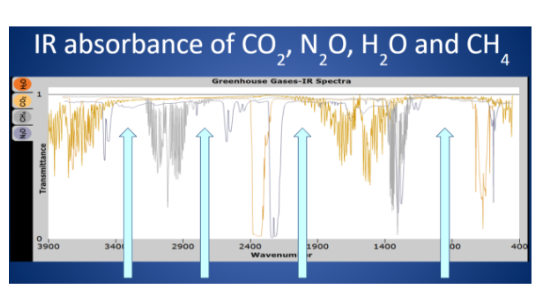
What are the four arrows on the image indicating, and what is the significance with respect to atmospheric temperatures?
1. the arrows indicate “windows” that allow thermal energy through to outer space
2. this particular circumstance cools atmospheric temperatures if thermal energy can escape to outer space from the atmosphere that’s heating from GHGs
84
New cards
Humans have introduced a class of GHGs that do not occur naturally on the planet. What are these compounds called, and what is the effect on the image above?
The class of compounds are refrigerants. They have effectively “closed” a window, trapping more IR energy in the atmosphere and precipitating the warming of the planet.
85
New cards
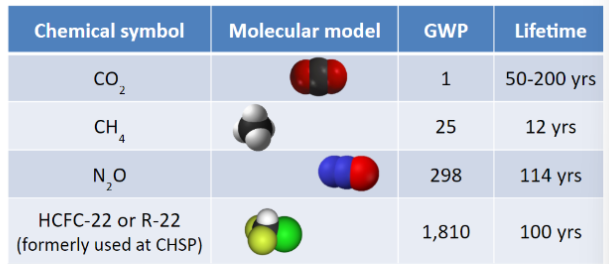
According to the table, 1 ton of N2O would trap the same amount of heat as _____ tons of CO2.
298
86
New cards
1. **Why might the GWP of a greenhouse gas** ***decrease*** **over time?**
it may have a shorter residence time in air
87
New cards
Water vapor is a greenhouse gas, and yet it doesn’t have a GWP. Explain why.
Water is extremely transient in nature. This means that the water vapor will have a residence time in air irregularly short in comparison to other gasses. Water will precipitate to water and leave the air readily as it moves through the various states of the water cycle
\
**Water vapor is here today and gone tomorrow. It doesn’t have a residence time in the atmosphere consistent enough to have a single GWP value.**
\
\
**Water vapor is here today and gone tomorrow. It doesn’t have a residence time in the atmosphere consistent enough to have a single GWP value.**
\
88
New cards
GHGs in the atmosphere are a good thing, but too much of a good thing can be a bad thing (a.k.a. everything in moderation). Explain what this means in connection to GHGs.
GHGs in the atmosphere are necessary to keep the planet at a temperature range that is suitable for complex life. However, too many GHGs and the planet begins to heat up which threatens the natural balance of many ecosystems (as we’re seeing).
89
New cards
1. **All fuels are not created equal.**
1. **List three fuels that are not fossil fuels**
biomass, nuclear, hydroelectric
90
New cards
Name three different products made from distilling and refining petroleum.
Options include refinery gases like methane, ethane, propane, butane; liquid fuels like gasoline, diesel, jet fuel, etc.; lubricants, asphalt, or other related substances (like plastics).
91
New cards
List one similarity and one difference between wood and coal
* **They are similar in that they are both burned but are different in the burning’s reason. usually there’s a utilitarian reason for wood burning and energy associated with fossil fuels burning in coal**
* **they both have origins in photosynthesis considering that wood is made from the life of a planet and its energy spent to grow through photosynthesis and coal is also stemmed from ancient rites of photosynthesis**
* **they both have origins in photosynthesis considering that wood is made from the life of a planet and its energy spent to grow through photosynthesis and coal is also stemmed from ancient rites of photosynthesis**
92
New cards
List the names or chemical formulas of two hydrocarbons.
Options include methane (CH4), ethane (C2H6), butane (C3H8), and other hydrocarbon compounds.
93
New cards
Describe how human beings’ use of fossil fuels connects to our third message about the carbon cycle: that where carbon ends up matters.
Full credit for connecting fossil fuel use to moving carbon on the planet, correctly describing an impact of that carbon moving. For example, by burning fossil fuels, humans are re-introducing carbon into the carbon cycle that has been locked away for millions of years. This extra carbon has to end up somewhere, and it’s currently accumulating in the atmosphere and oceans which is leading to issues of climate change, ocean acidification, etc.
94
New cards
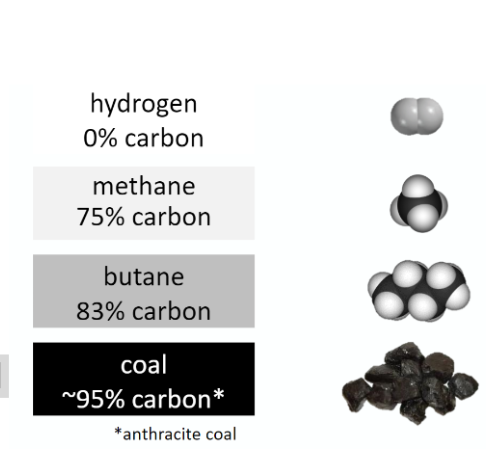
1. **Based on the image, why is methane considered an improvement over coal with respect to energy use and climate change?**
1. **The carbon content of methane is around twenty percent lower in methane. If this is used over coal, the amount of carbon (in co2 form) allowed into the atmosphere will be significantly less**
95
New cards
1. **Write the chemical formula for the reaction that caused the Hindenburg tragedy.**
hydrogen (H2) + oxygen (O2) → water vapor (H2O)
96
New cards
Which three fuels provide the most amount of energy? List them starting from the top.
Petroleum (highest), natural gas, coal (lowest).
97
New cards
Name the 5 criteria air pollutants (both the full name and shorthand chemical formulas).
1. **fine particles – PM_2.5 and PM_10**
2. **nitrogen oxides – NO and NO_2**
3. **carbon monoxide – CO**
4. **sulfur dioxide (SO_2)**
5. **ozone – O_3**
98
New cards
\n
1. **What is meant by the term “criteria air pollutant”?**
1. **What is meant by the term “criteria air pollutant”?**
An air pollutant is a regulated substance that is used to determine air quality based on criteria that relate to human health.
99
New cards

Rank these four air pollutants in the image from least to most toxic based on their AQI 101 ratings
**co, no2, so2, o3**
100
New cards
Name and describe the weather phenomenon that exacerbated the effects of the Donora smog event.
The phenomenon is called an air inversion, where cold air is trapped under warm air and accumulates air pollution.