Chem 40A functional groups
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
49 Terms
R
Some unspecified carbon containing group
X
Some unspecified halogen
carbonyl group
C=O
alkane
single bonds
sp^3 carbons
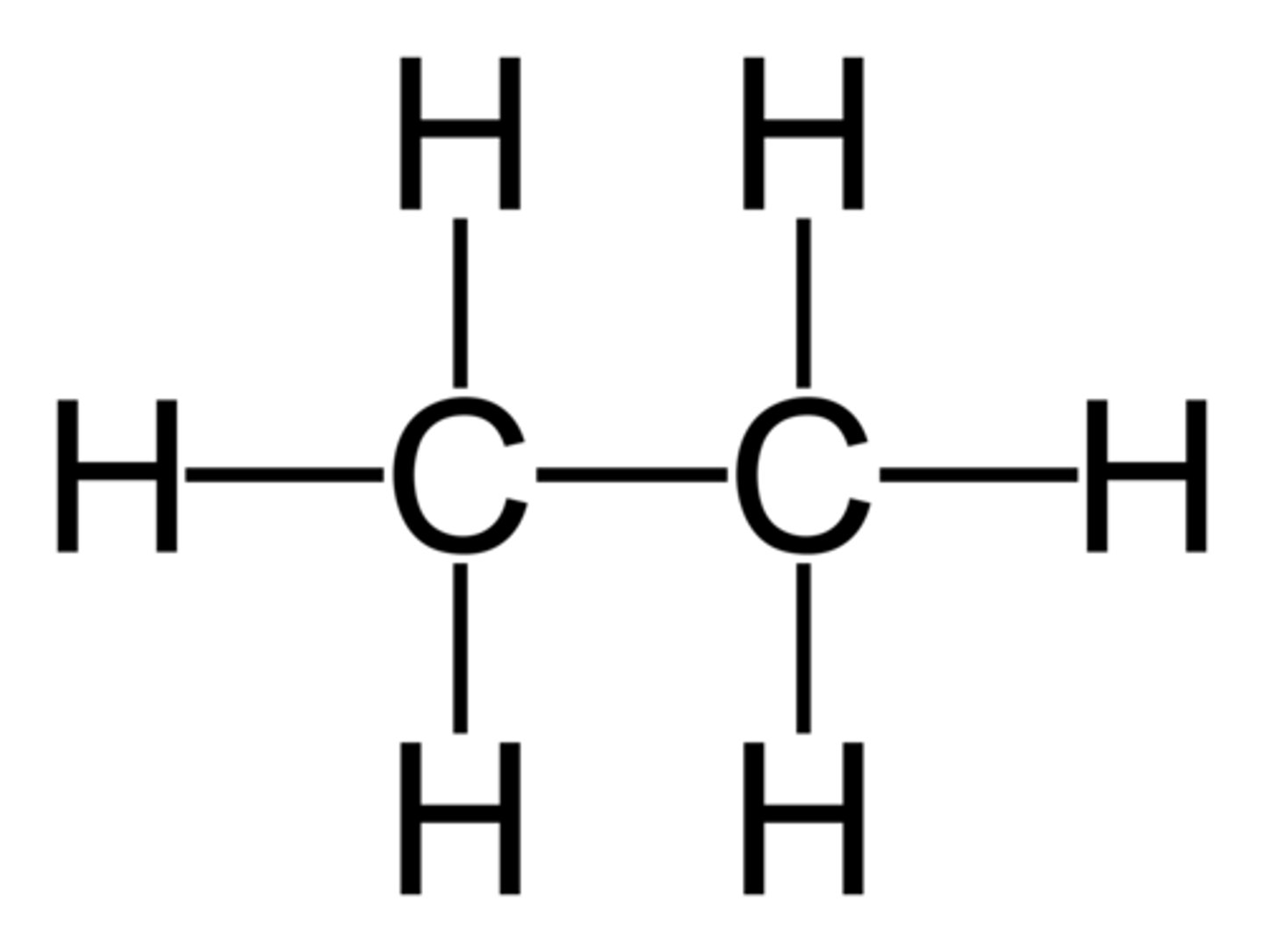
alkene
double bond
sp2 carbons
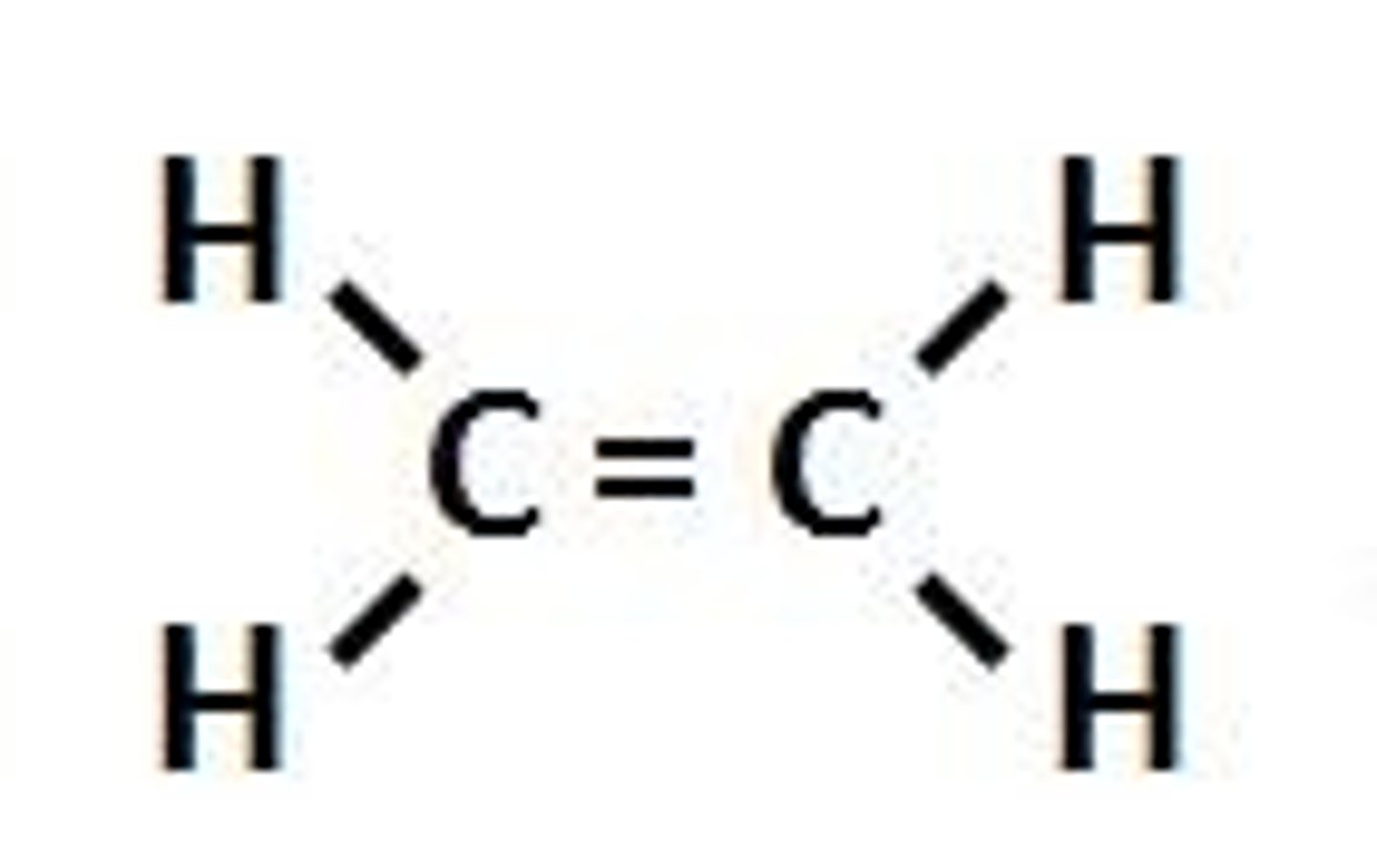
alkyne
triple bond
sp carbons
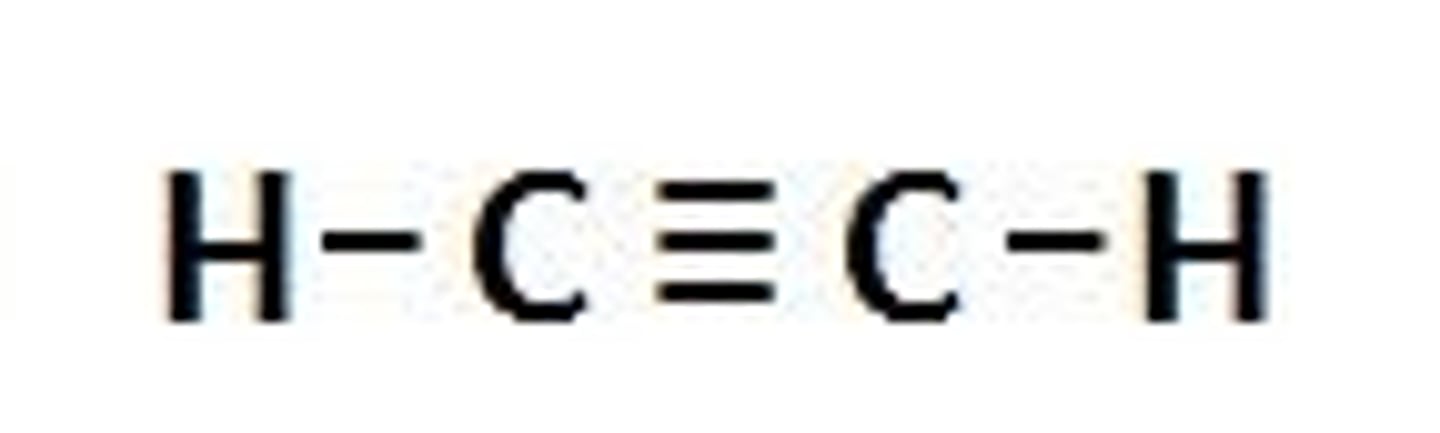
aromatic
contains a benzene ring
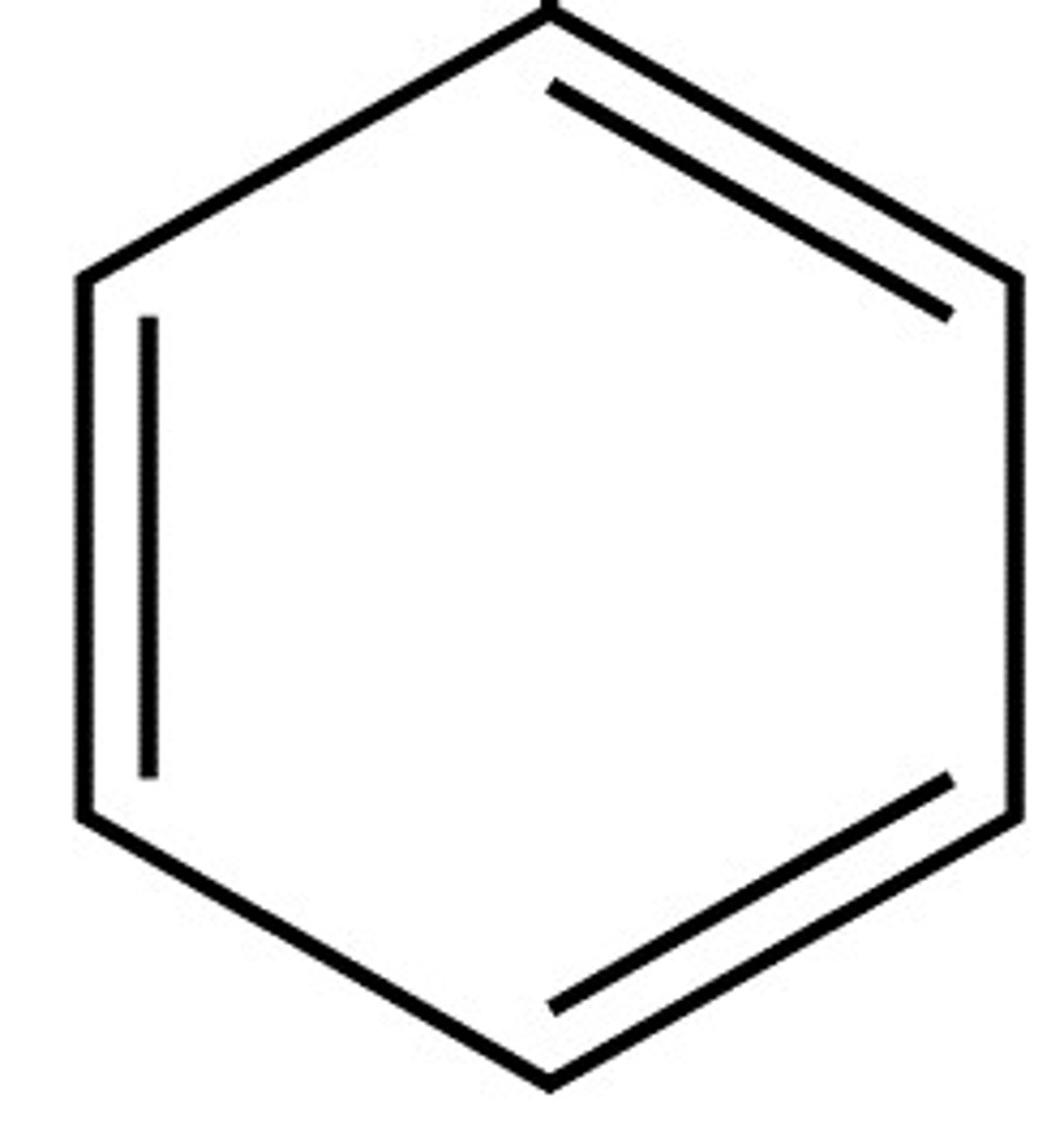
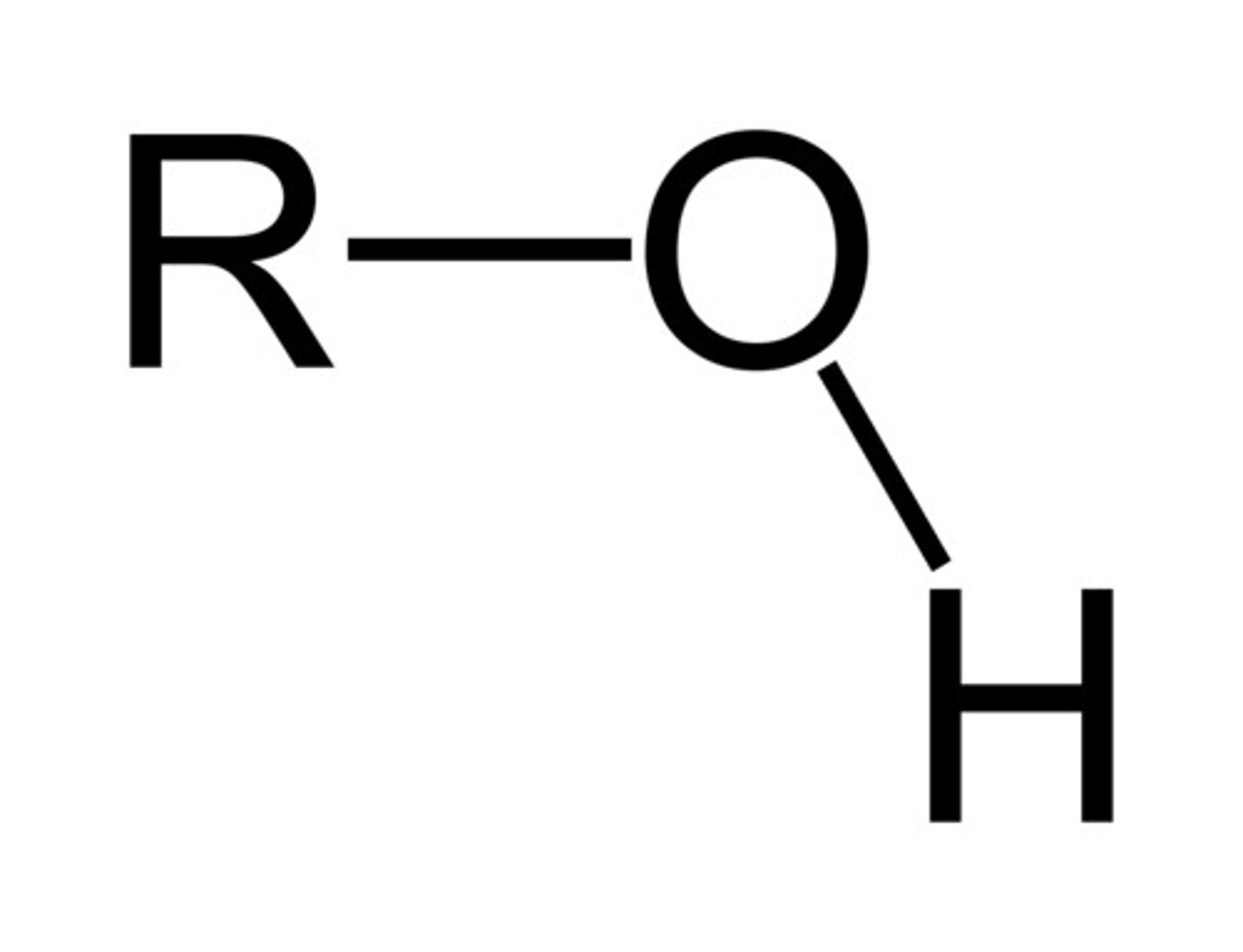
alcohol
R-OH
only sp3 hybridized carbons
thioalcohol (thiol)
R-SH
only sp3 hybridized carbons
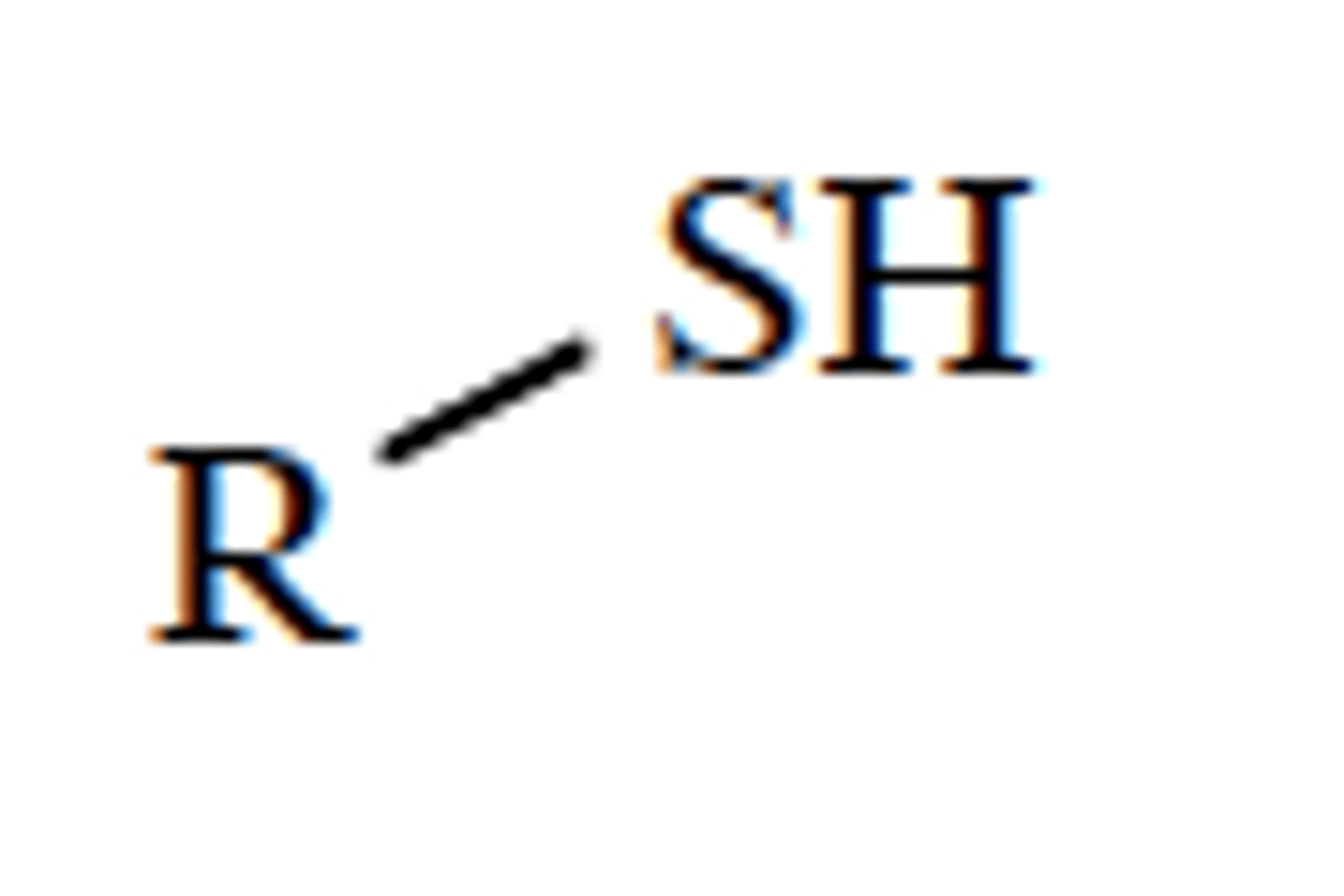
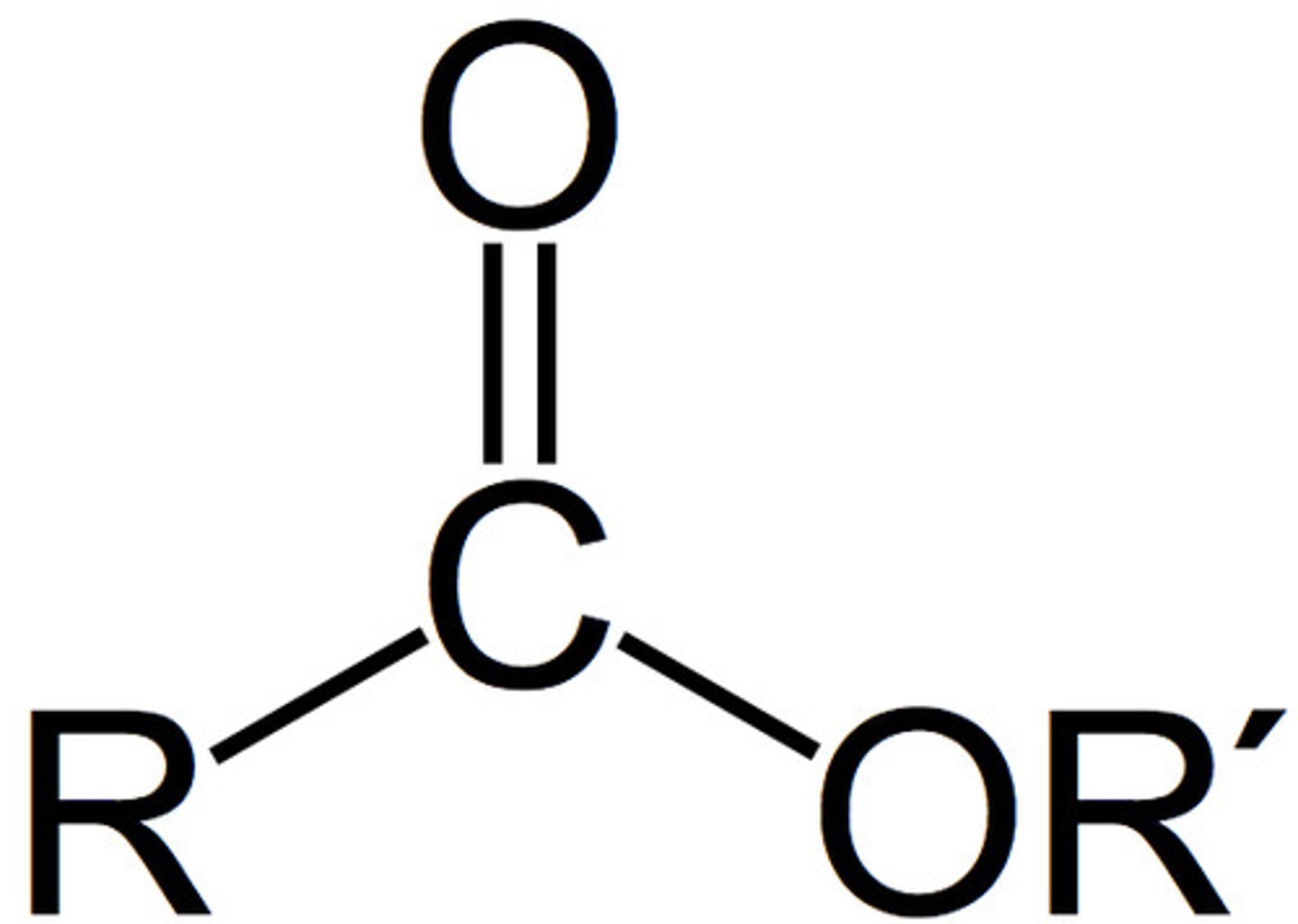
ester
RCOOR
only sp3 hybridized carbons
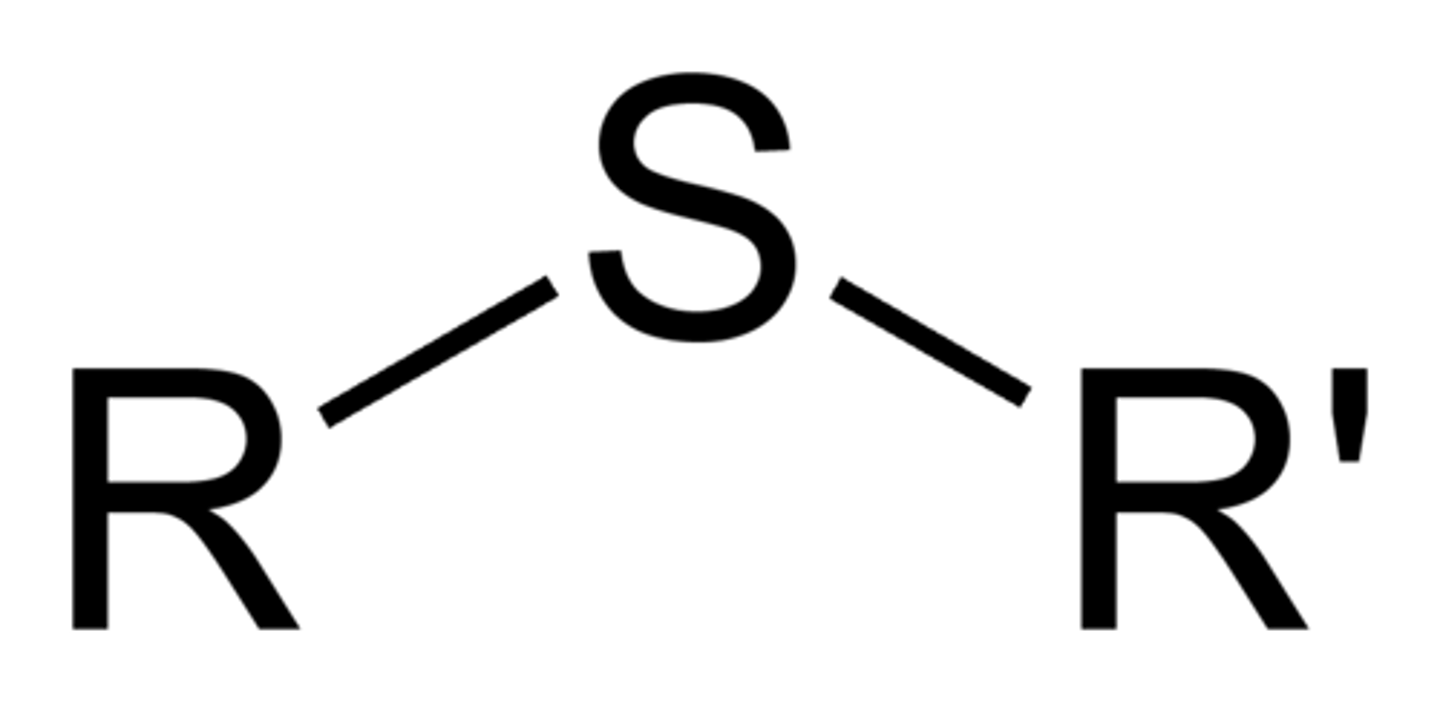
sulfide
R-S-R
only sp3 hybridized carbons
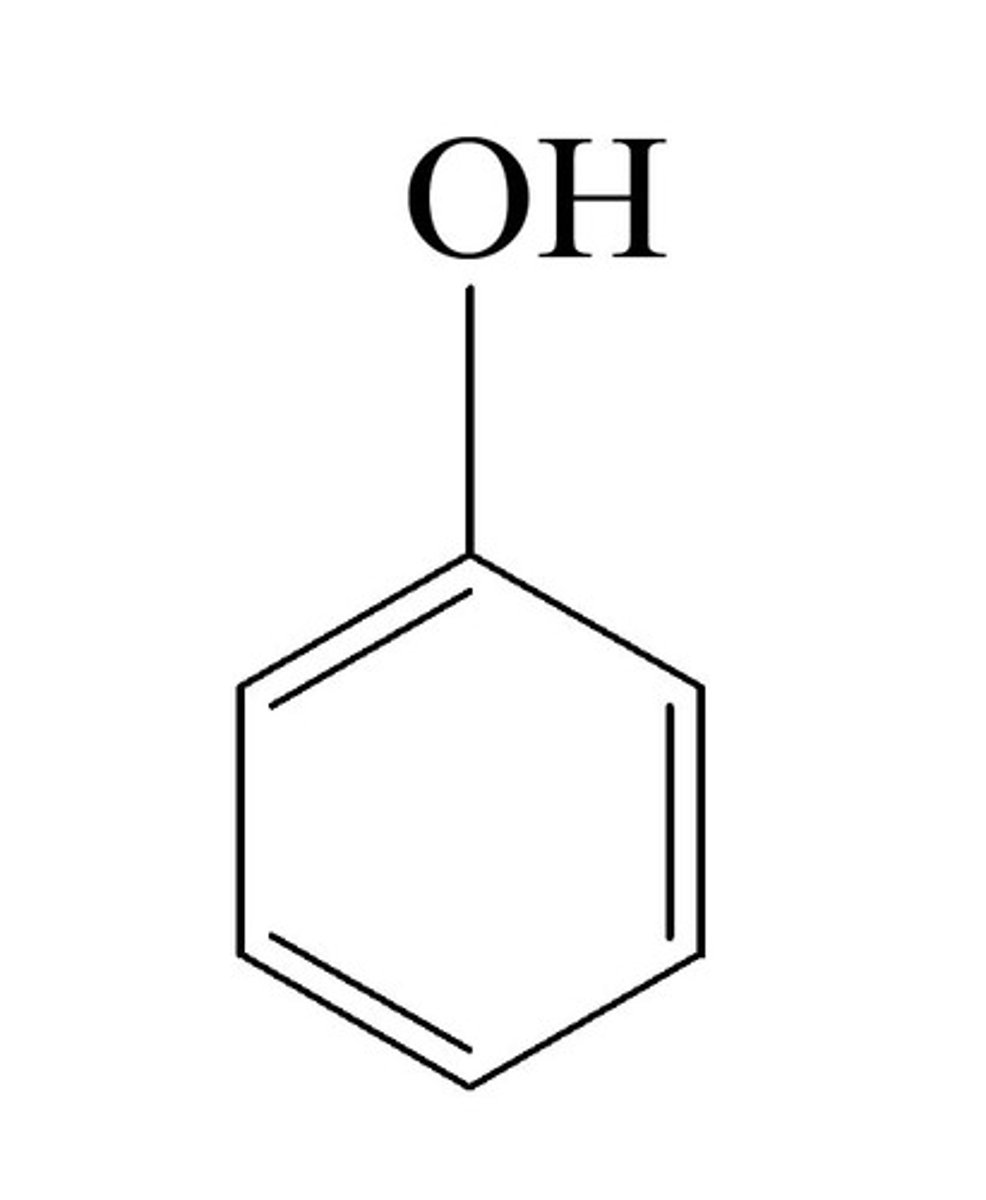
phenol
only sp3 hybridized carbons
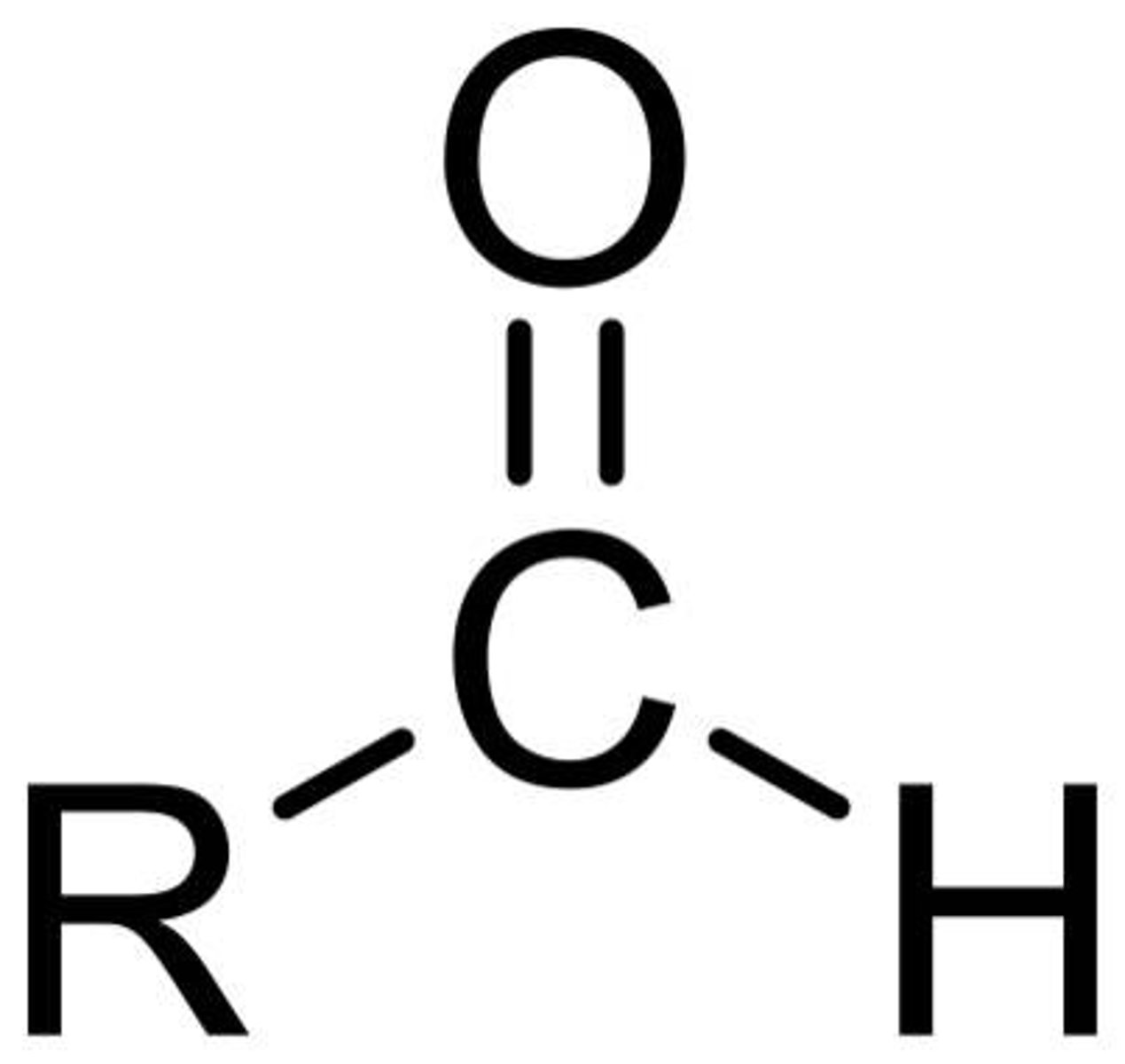
aldehyde
RCHO
only sp2 hybridized carbons
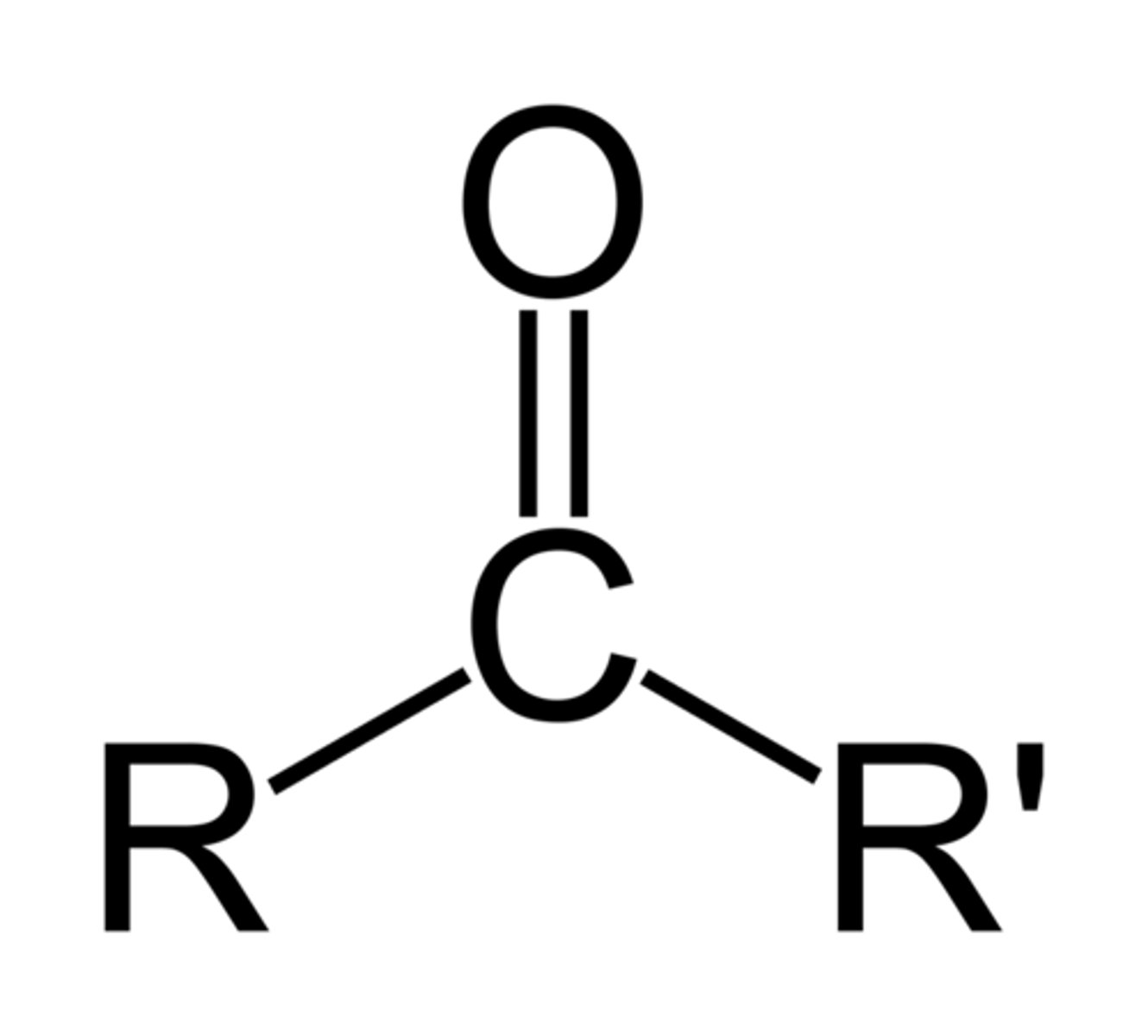
ketone
RCOR
only sp2 hybridized carbons
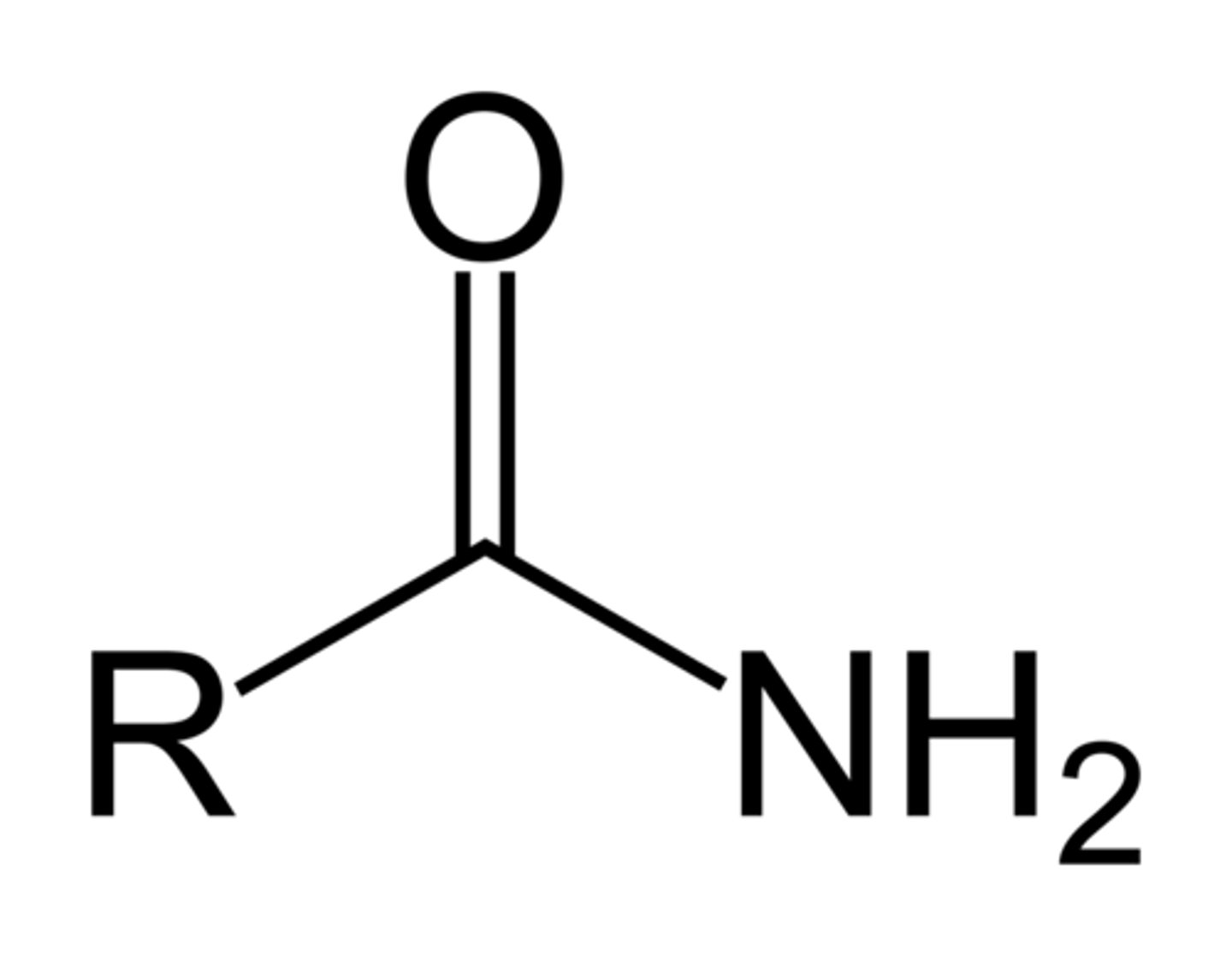
amide
RCONH2, RCONHR, RCONHR2
Primary amide
When a nitrogen is bonded to a carbonyl group and two Hs
Secondary Amide
Nitrogen is bound to a carbonyl group, R and H
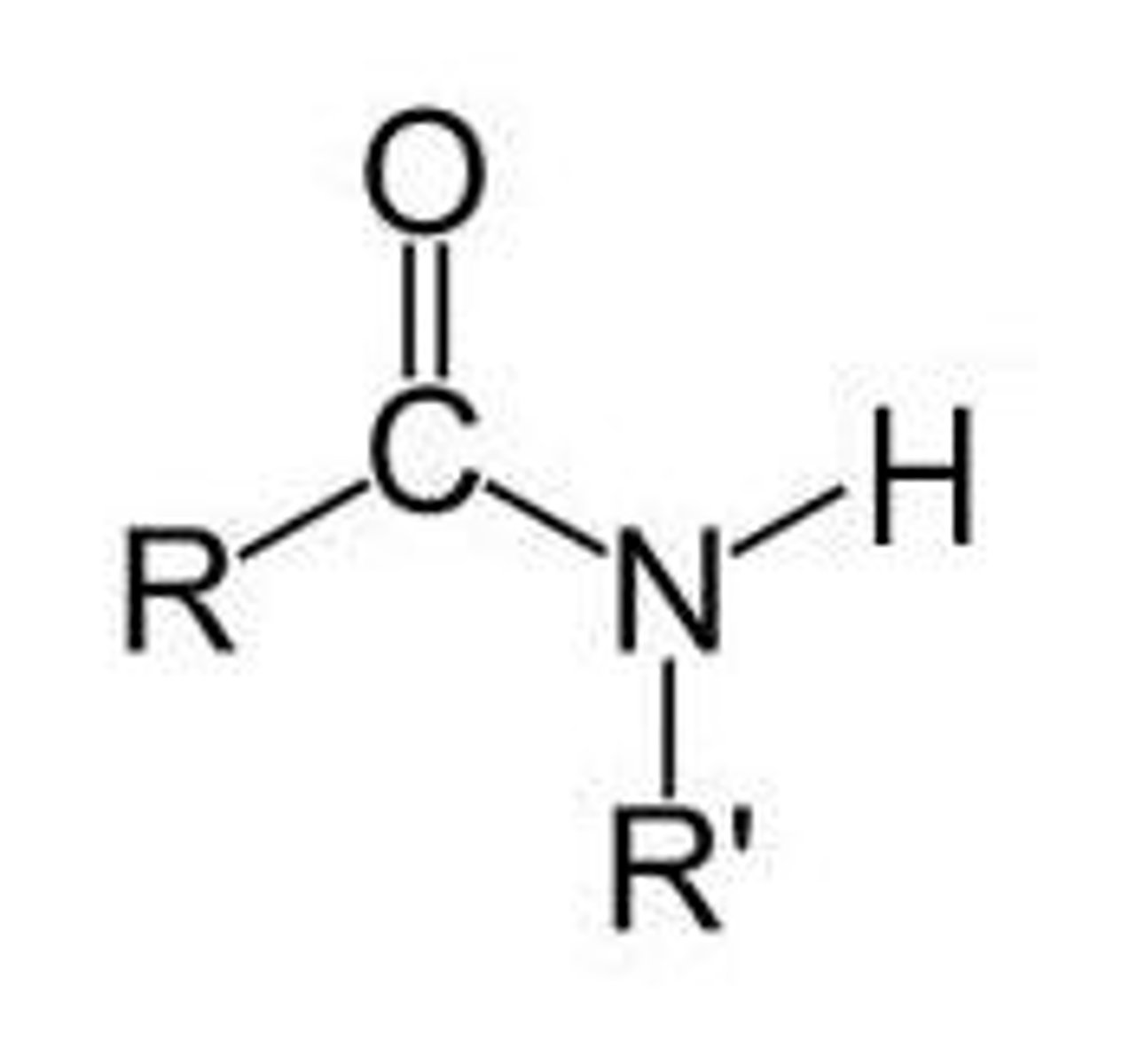
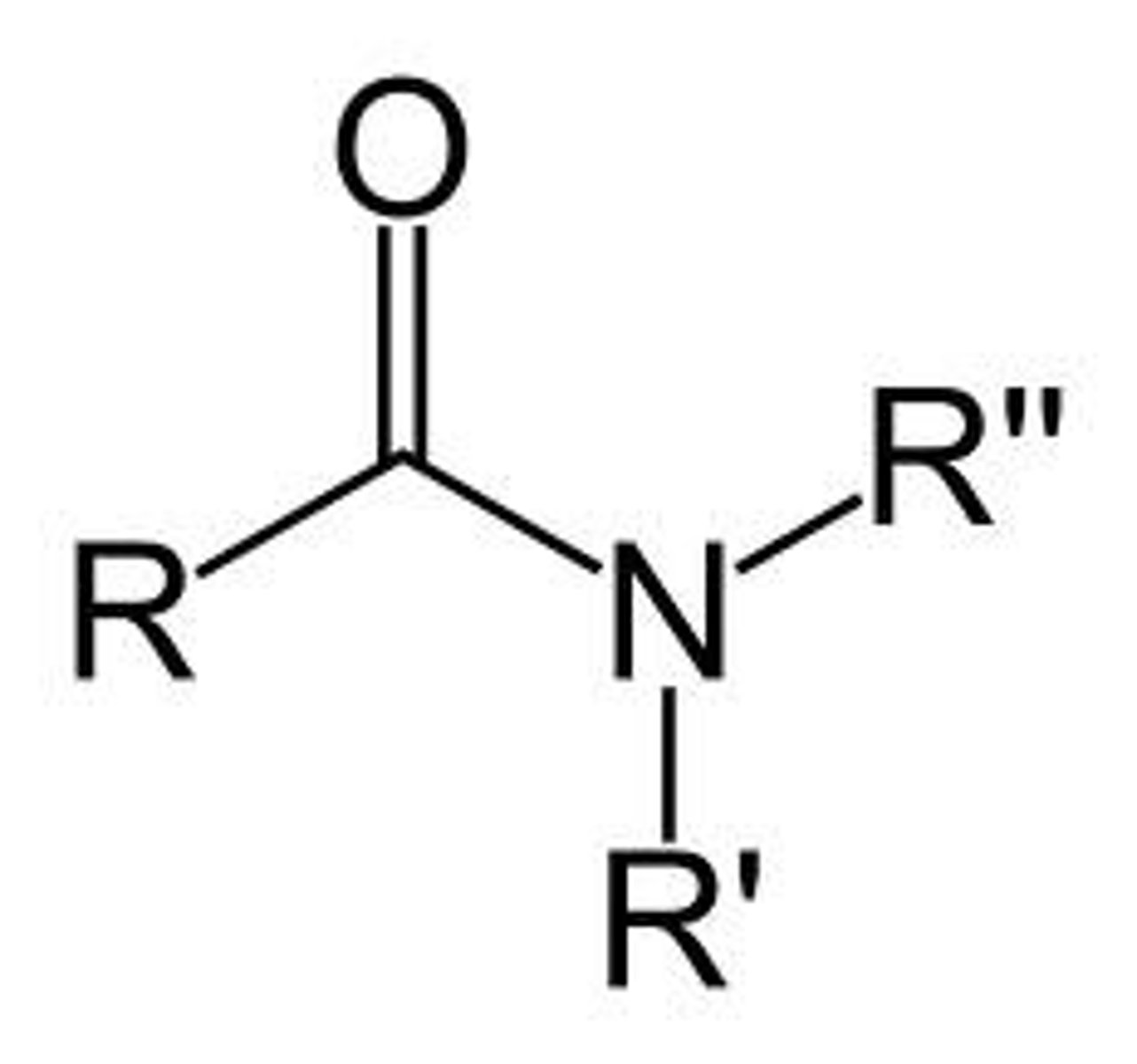
Tertiary Amide
Nitrogen is bound to a carbonyl group, and two Rs
amine
RNH2, RNHR, R3N
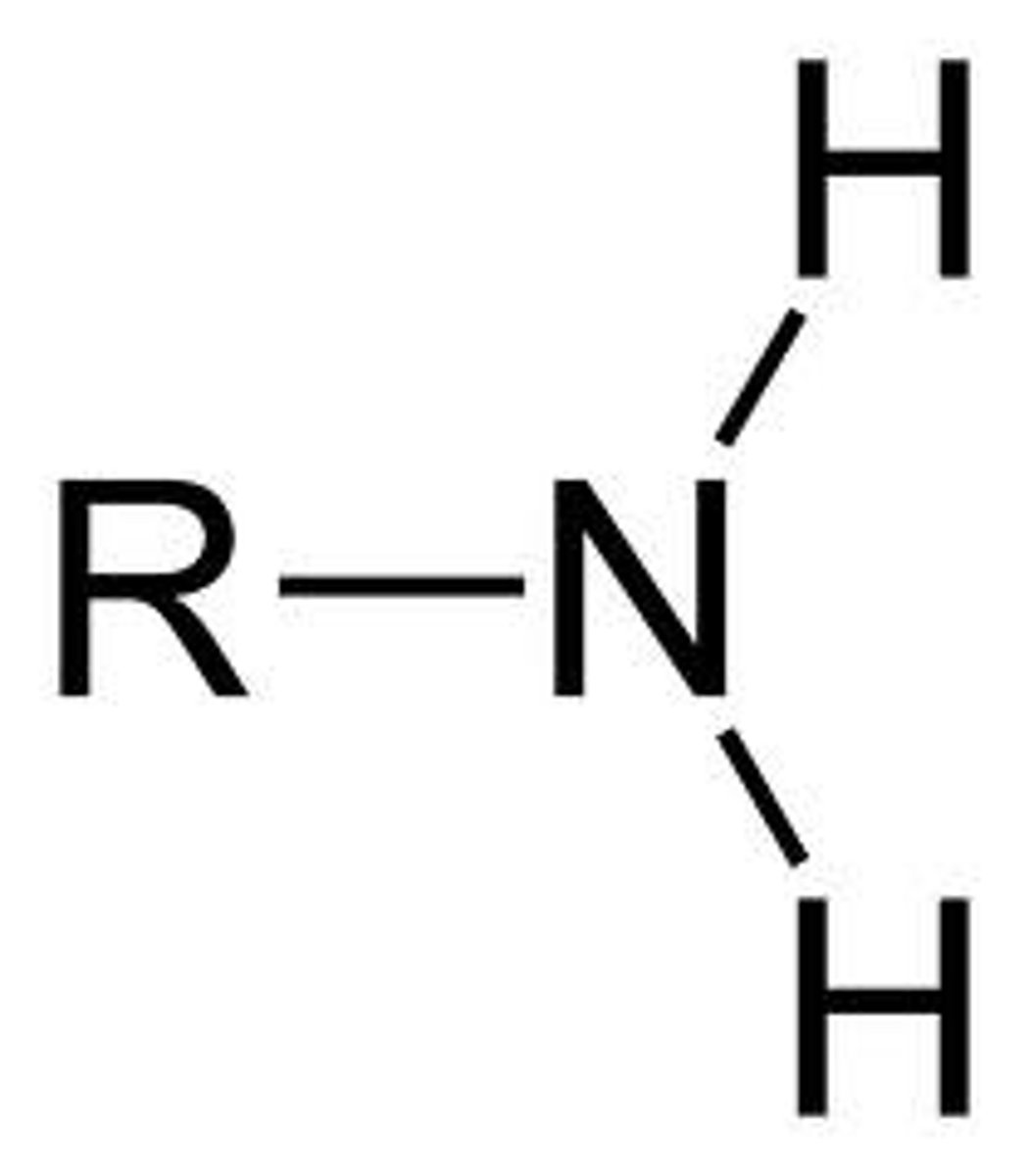
Primary amine
Nitrogen has two H's and one R
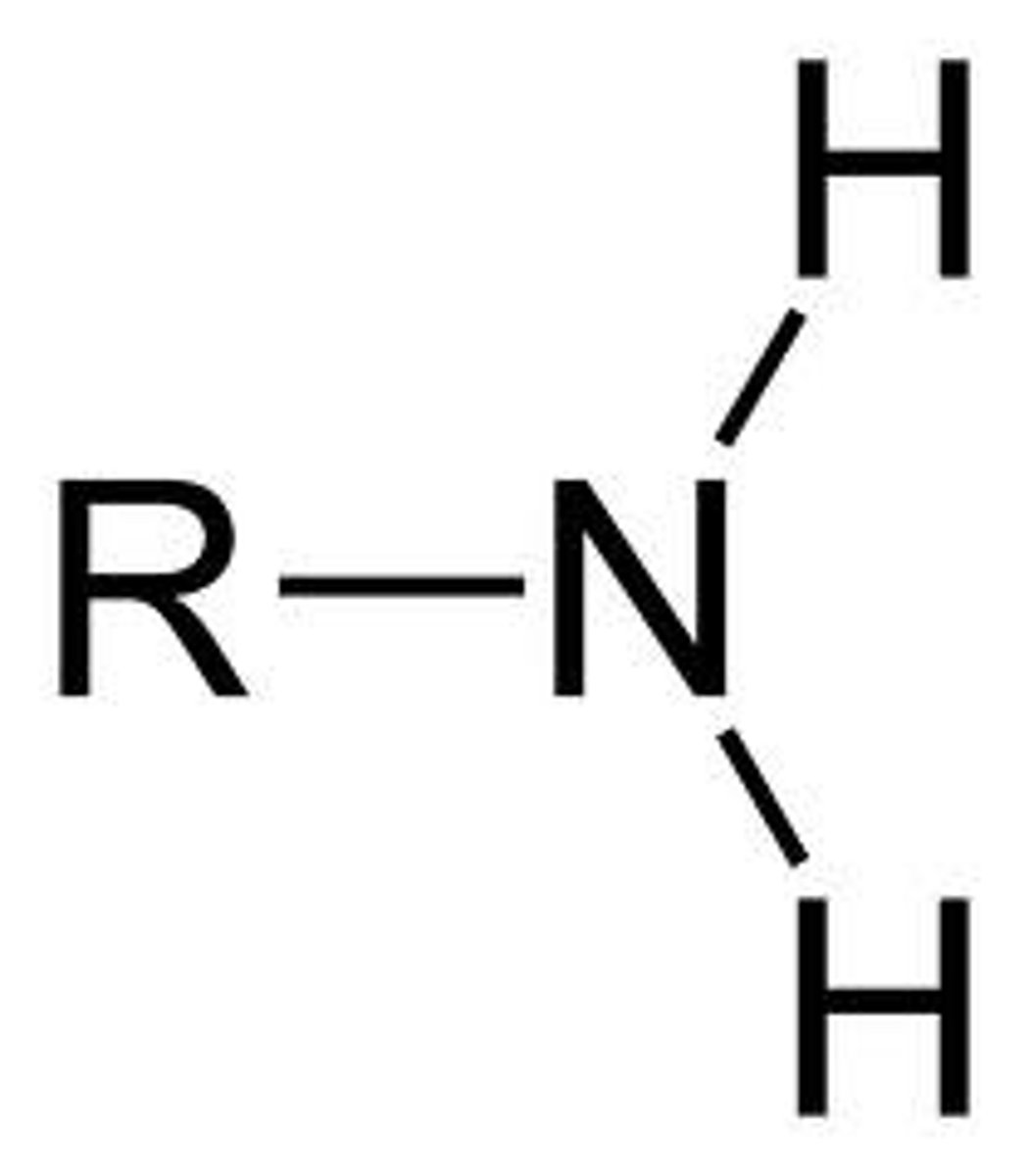
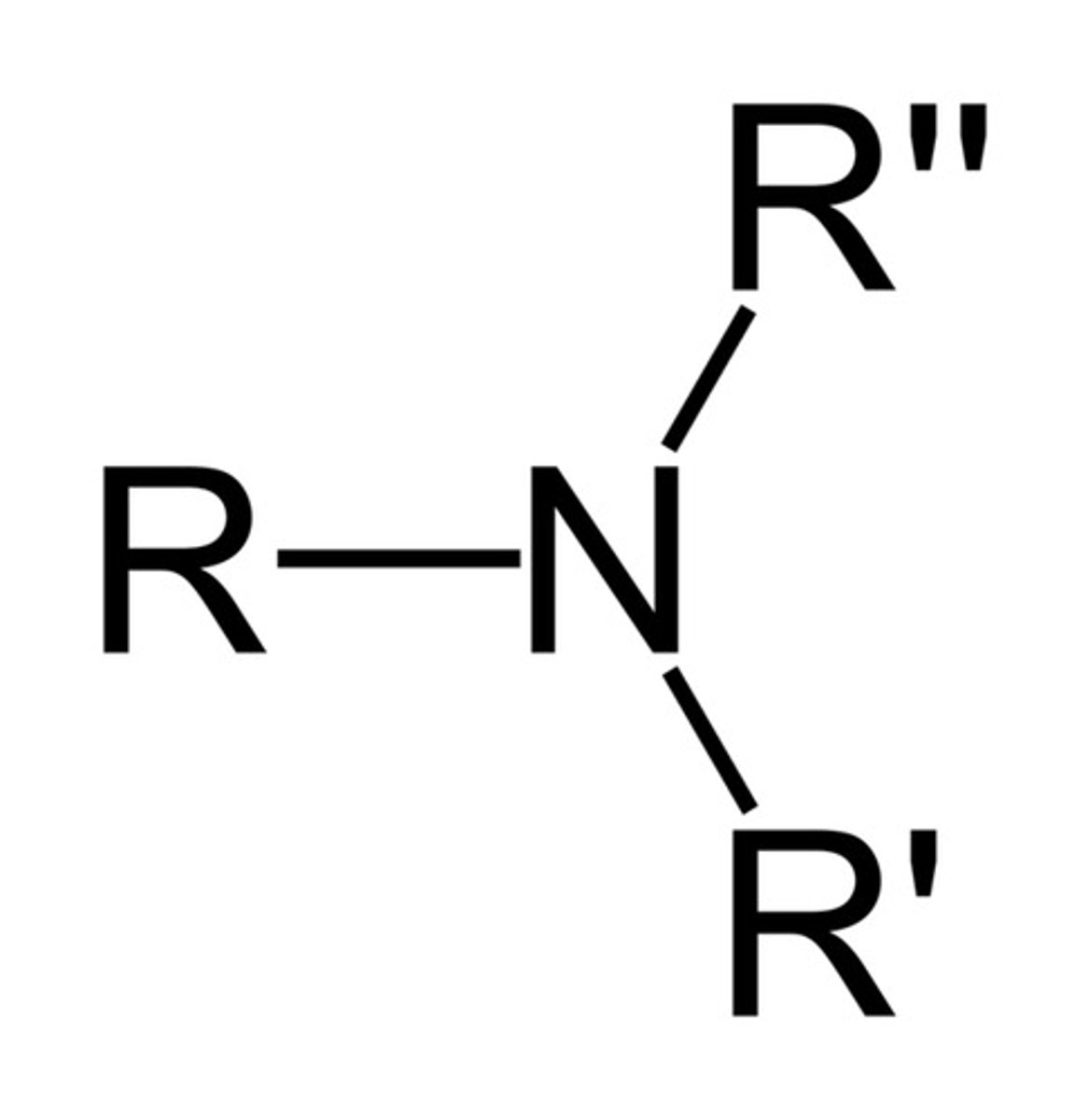
teritary amine
Nitrogen bonded to three Rs
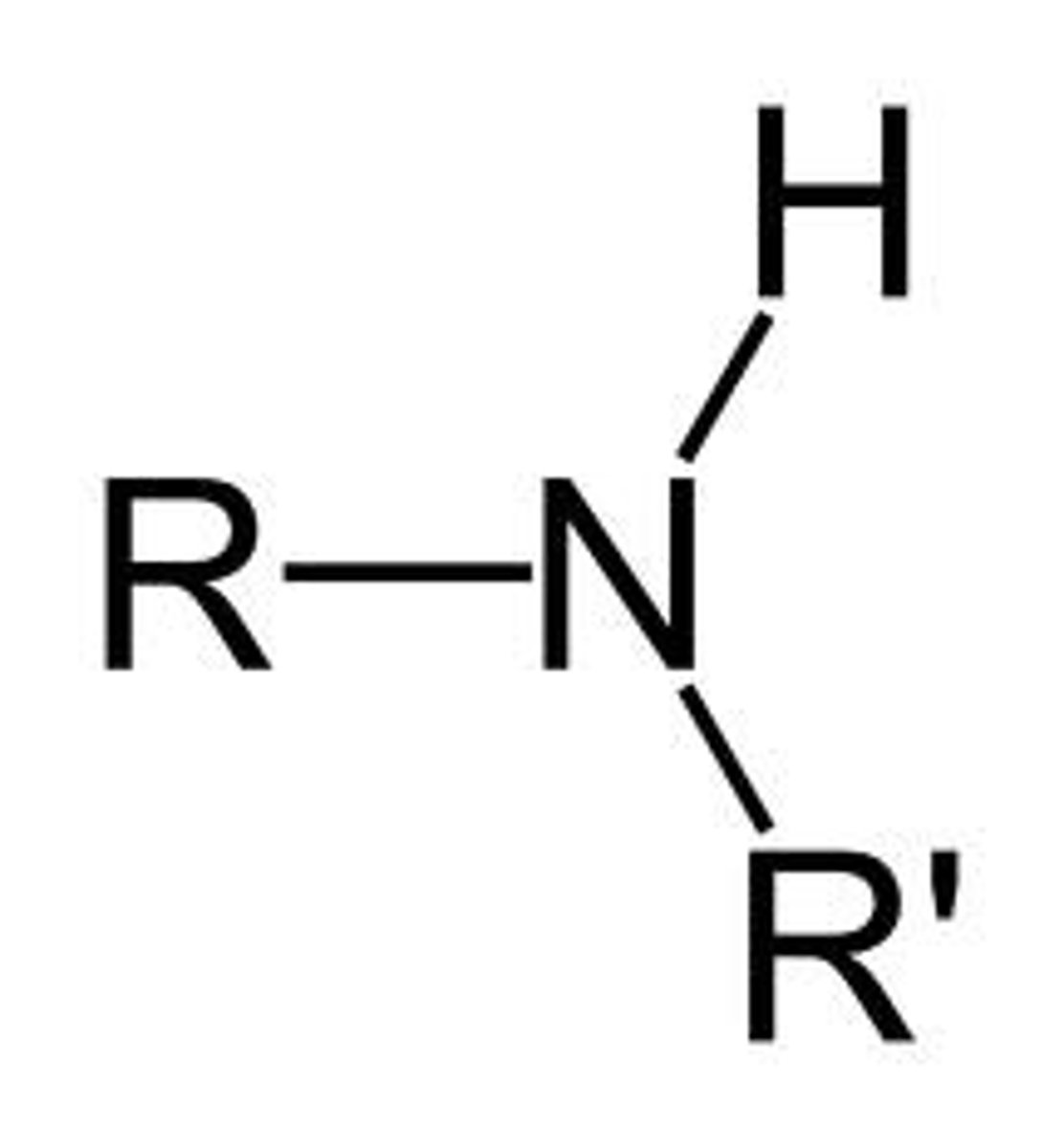
Secondary amine
Nitrogen has one H and two Rs
ether
C-O-C
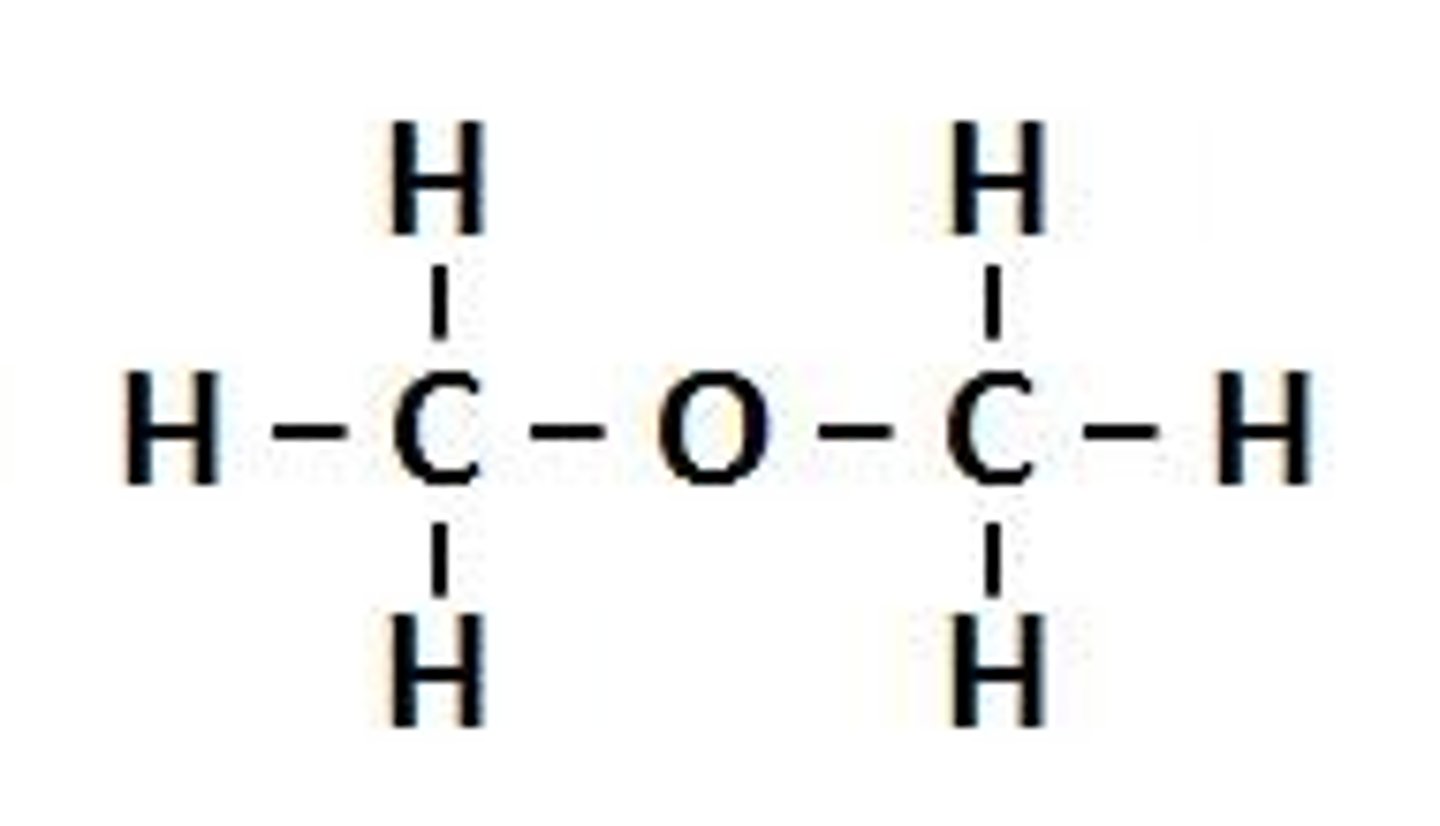
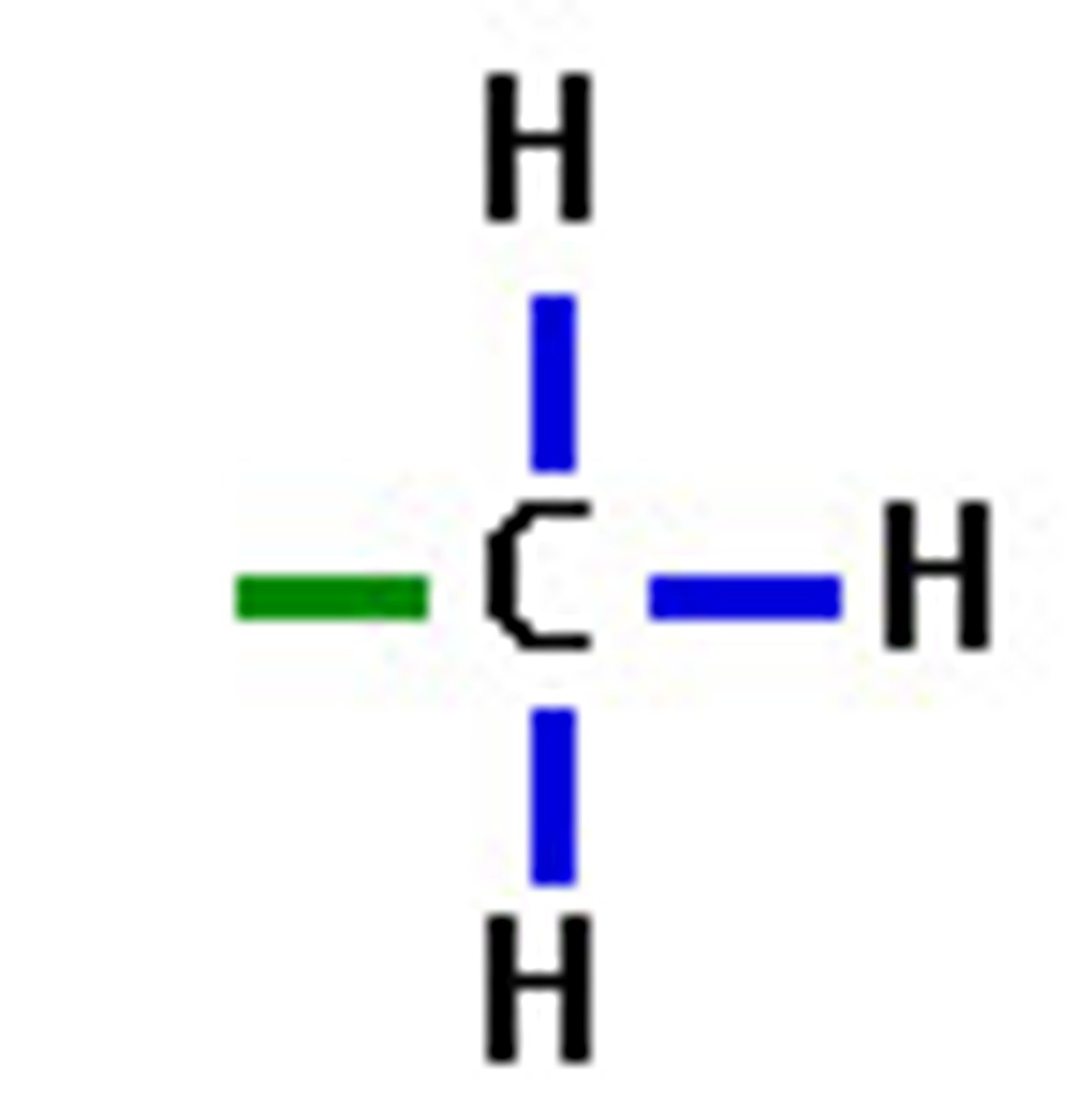
methyl
CH3
ethyl
CH3CH2
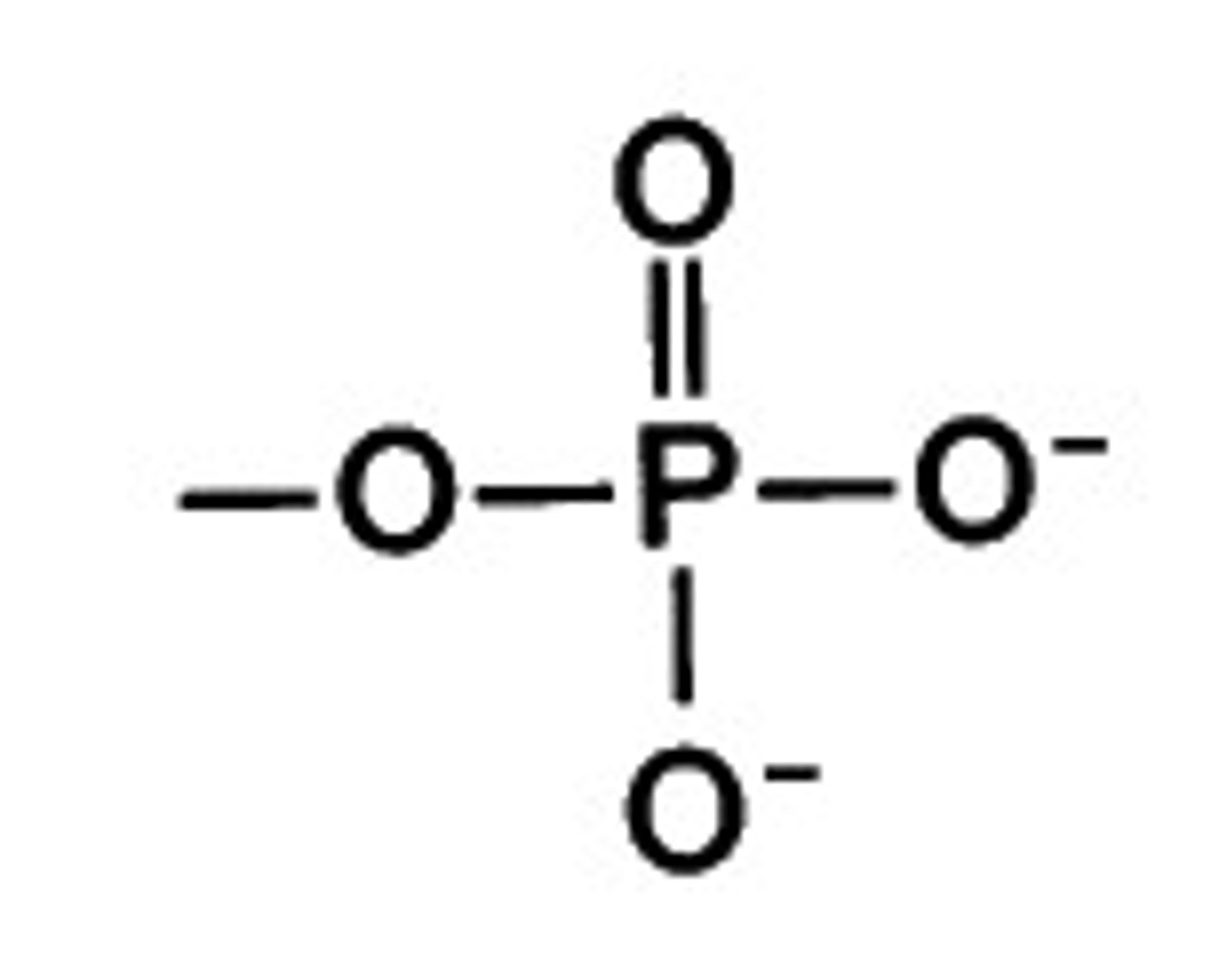
Phosphate ion
PO4 3-
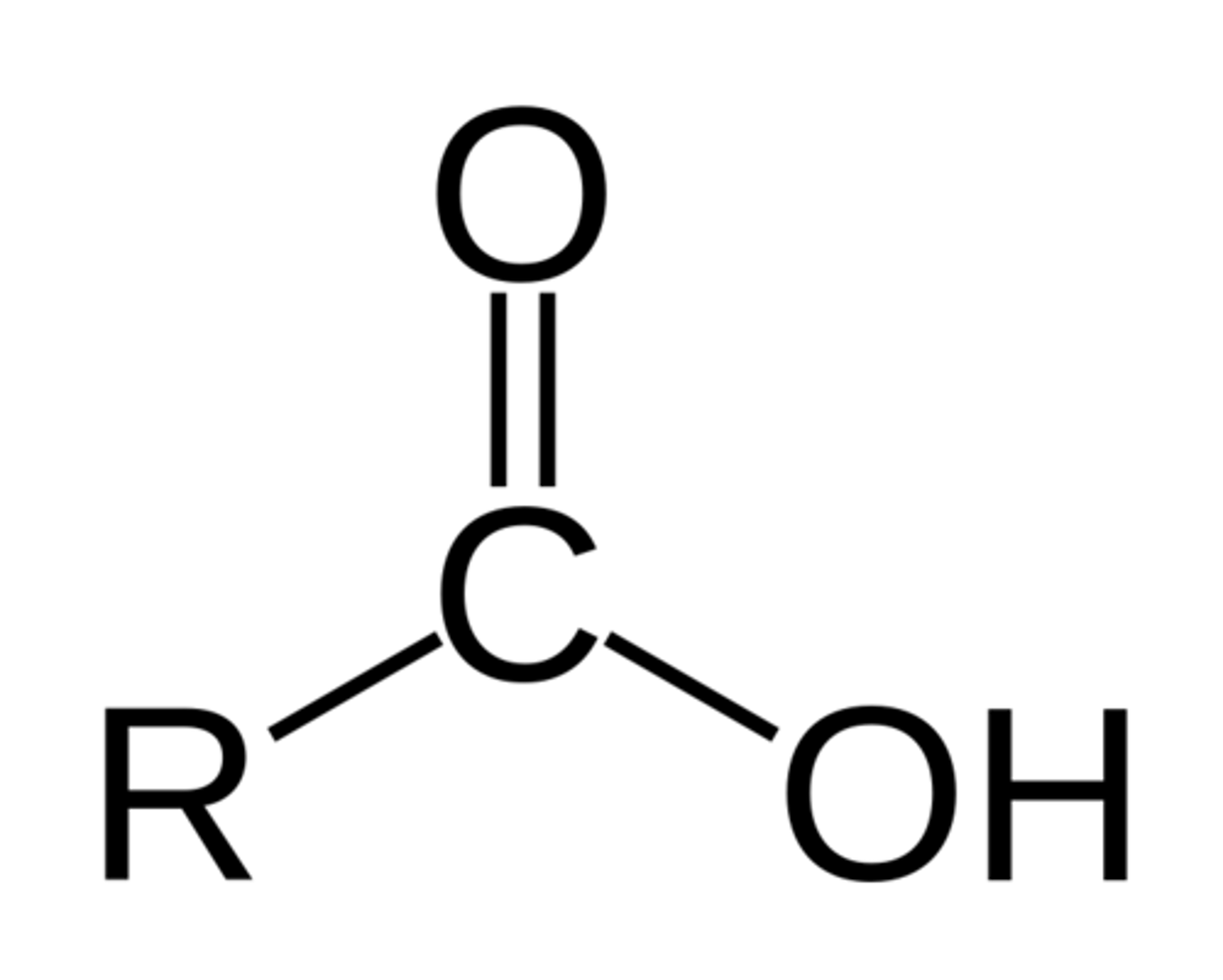
carbonxylic acid
COOH
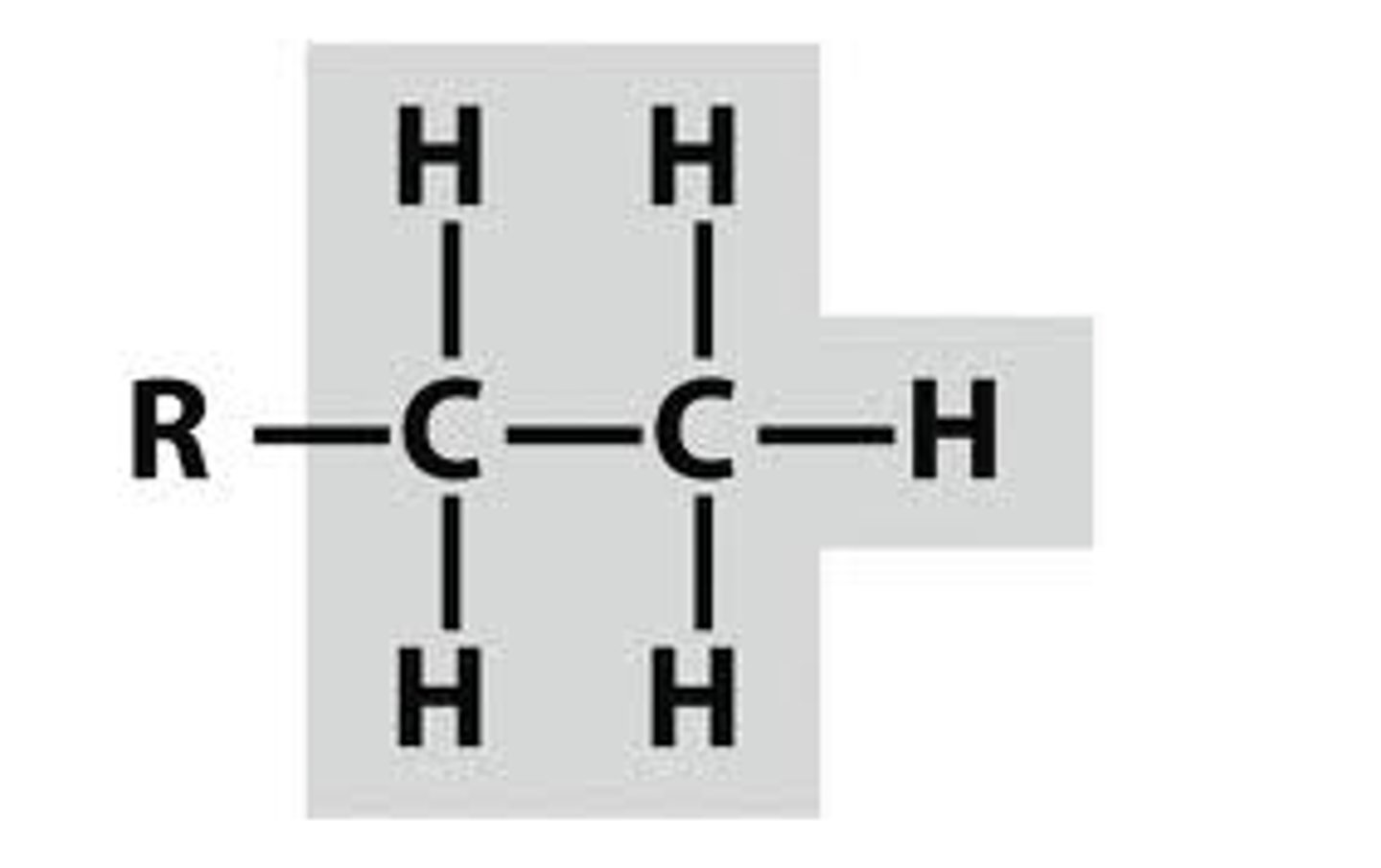
n-propyl
CH3CH2CH2
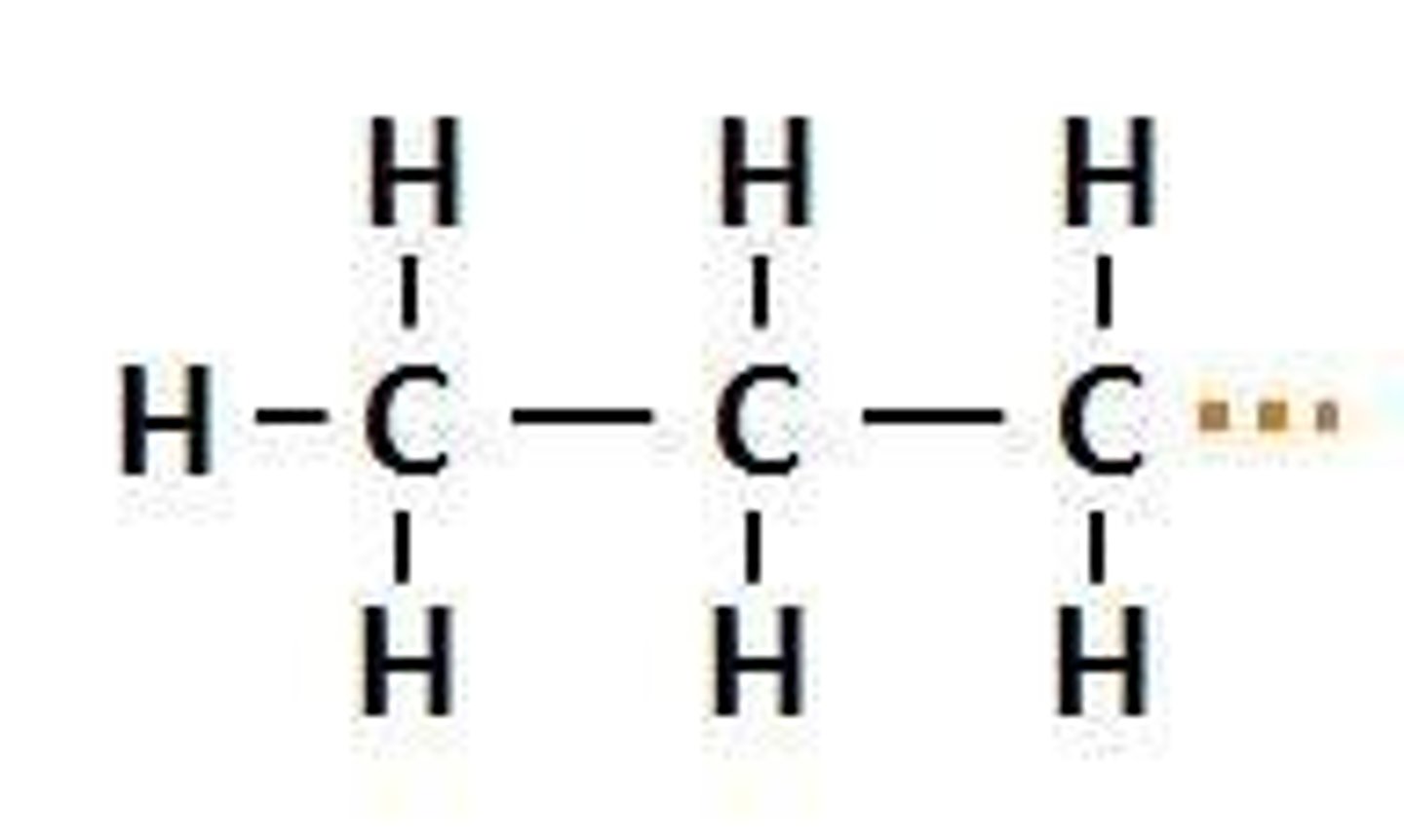
n-butyl
CH3CH2CH2CH2
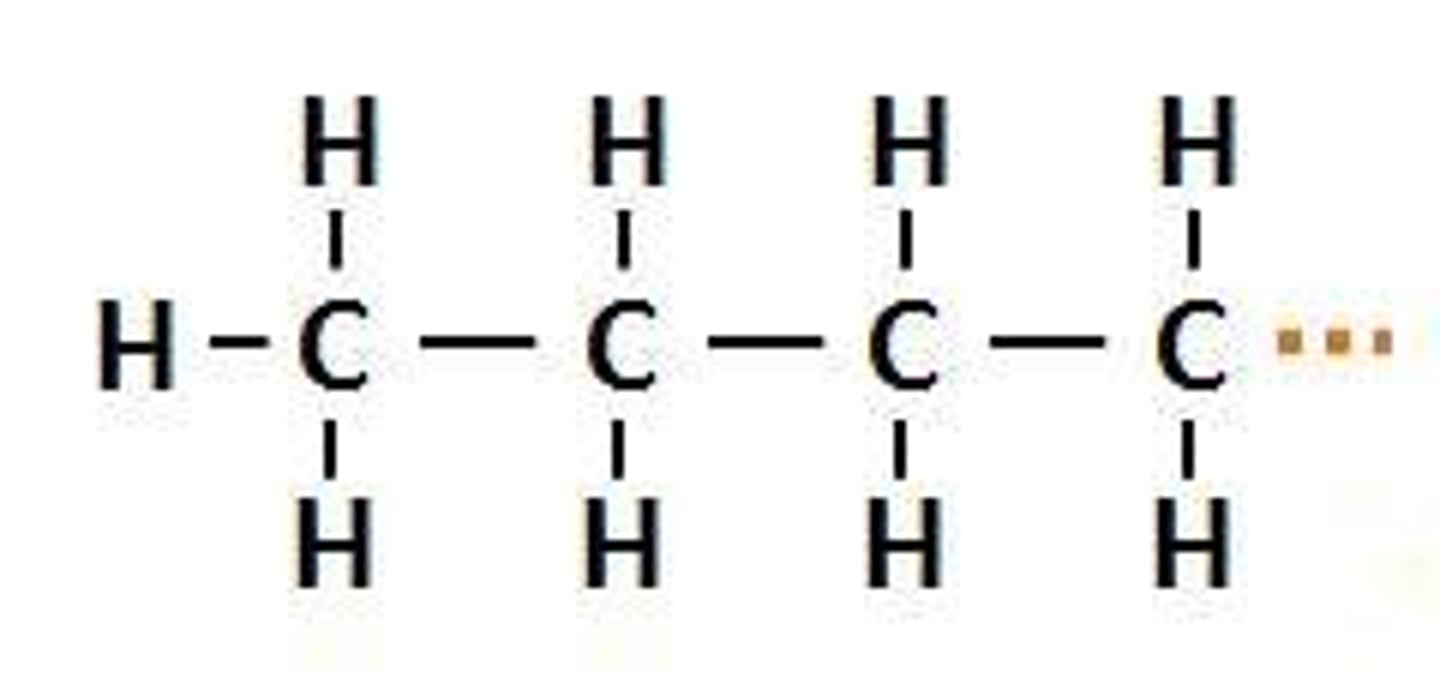
tert-butyl
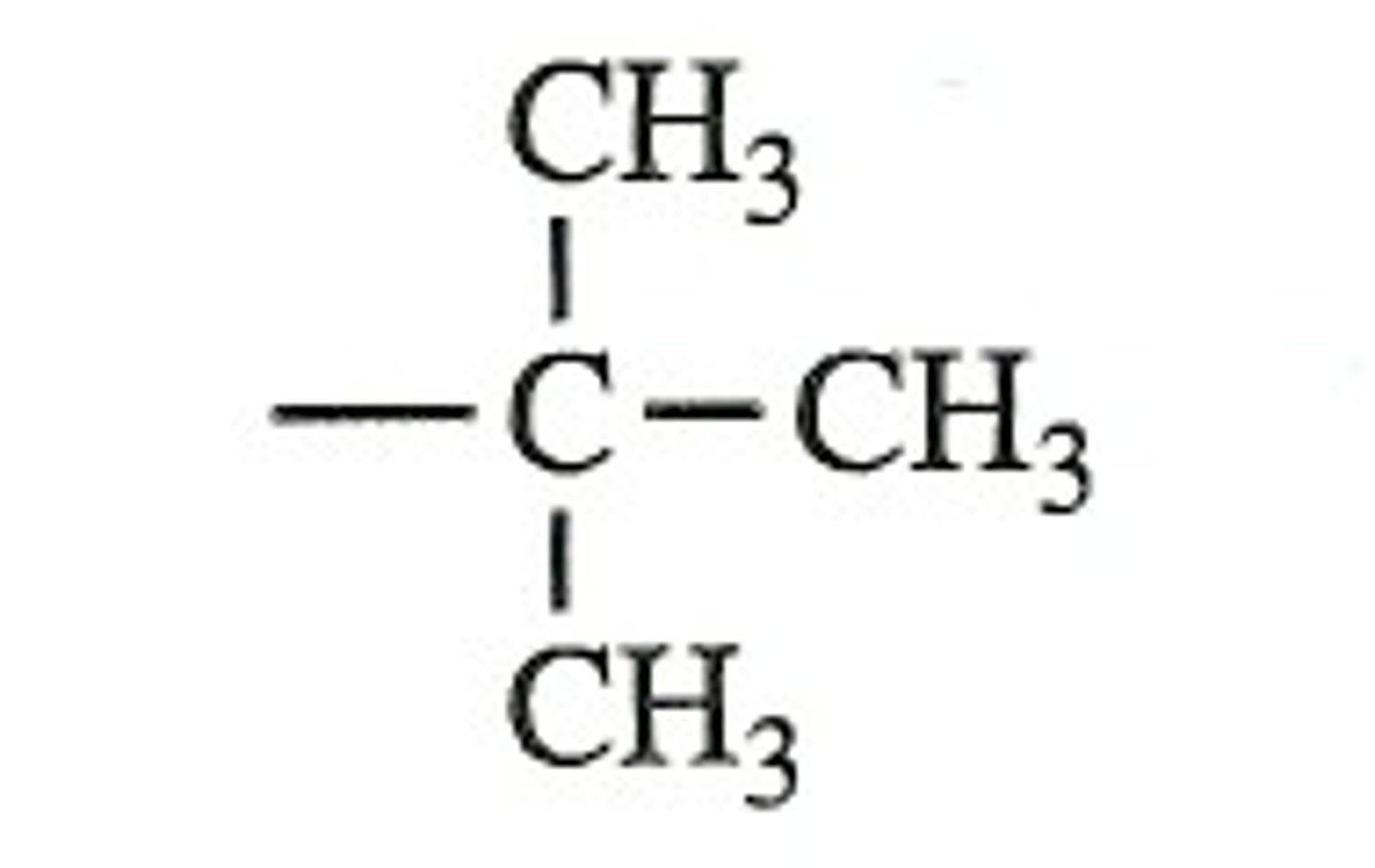
iso propyl
on the second carbon
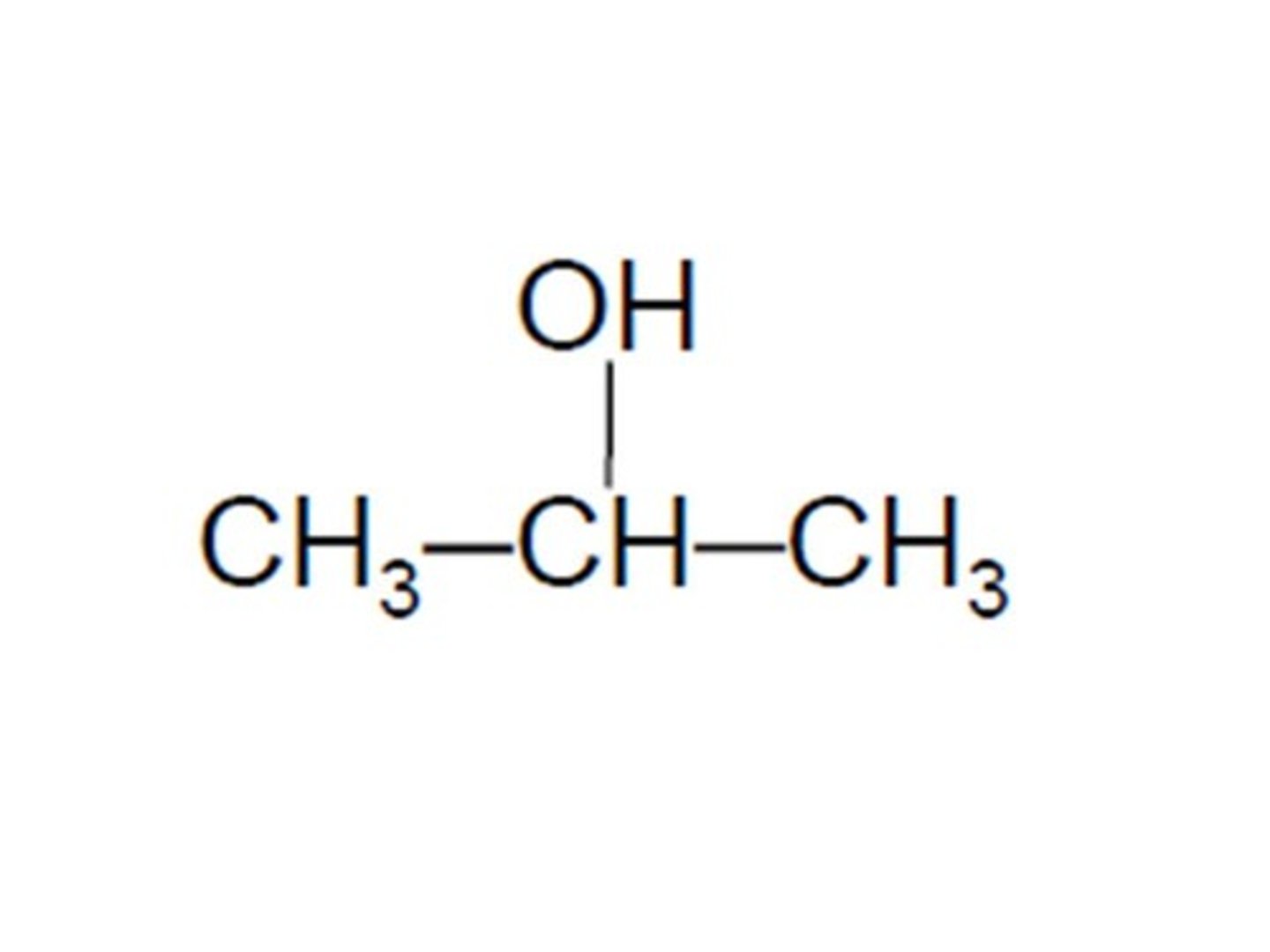
isobutane
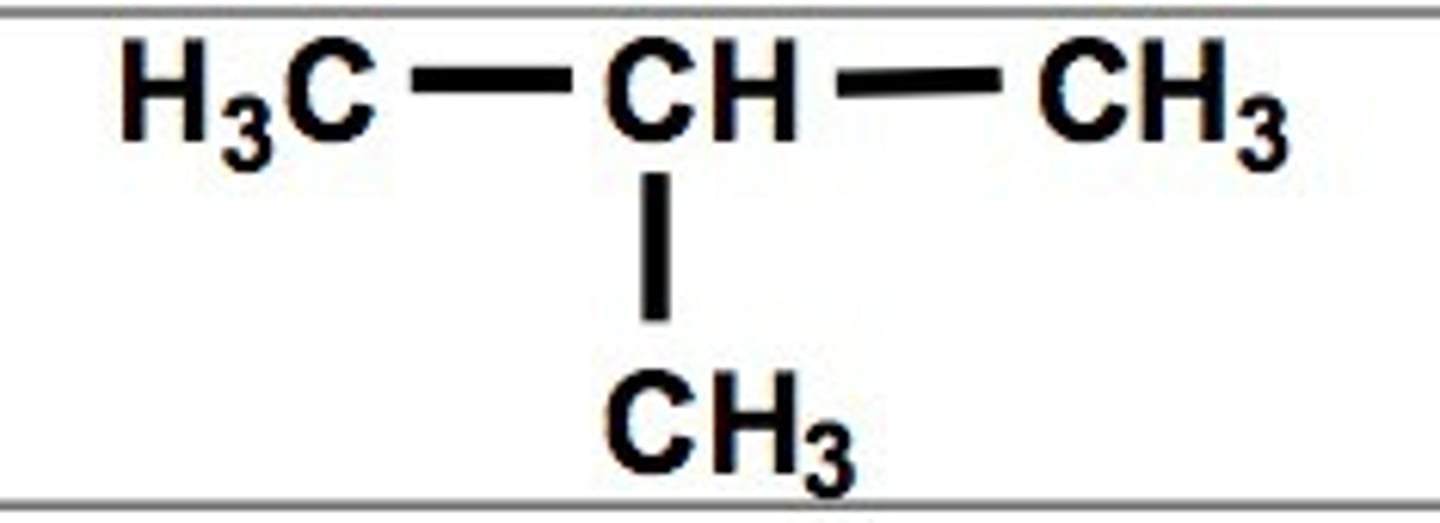
butane, on second C
2n +2
n=amount of carbons, to figure out number of hydeorgens
methane
1 Carbon
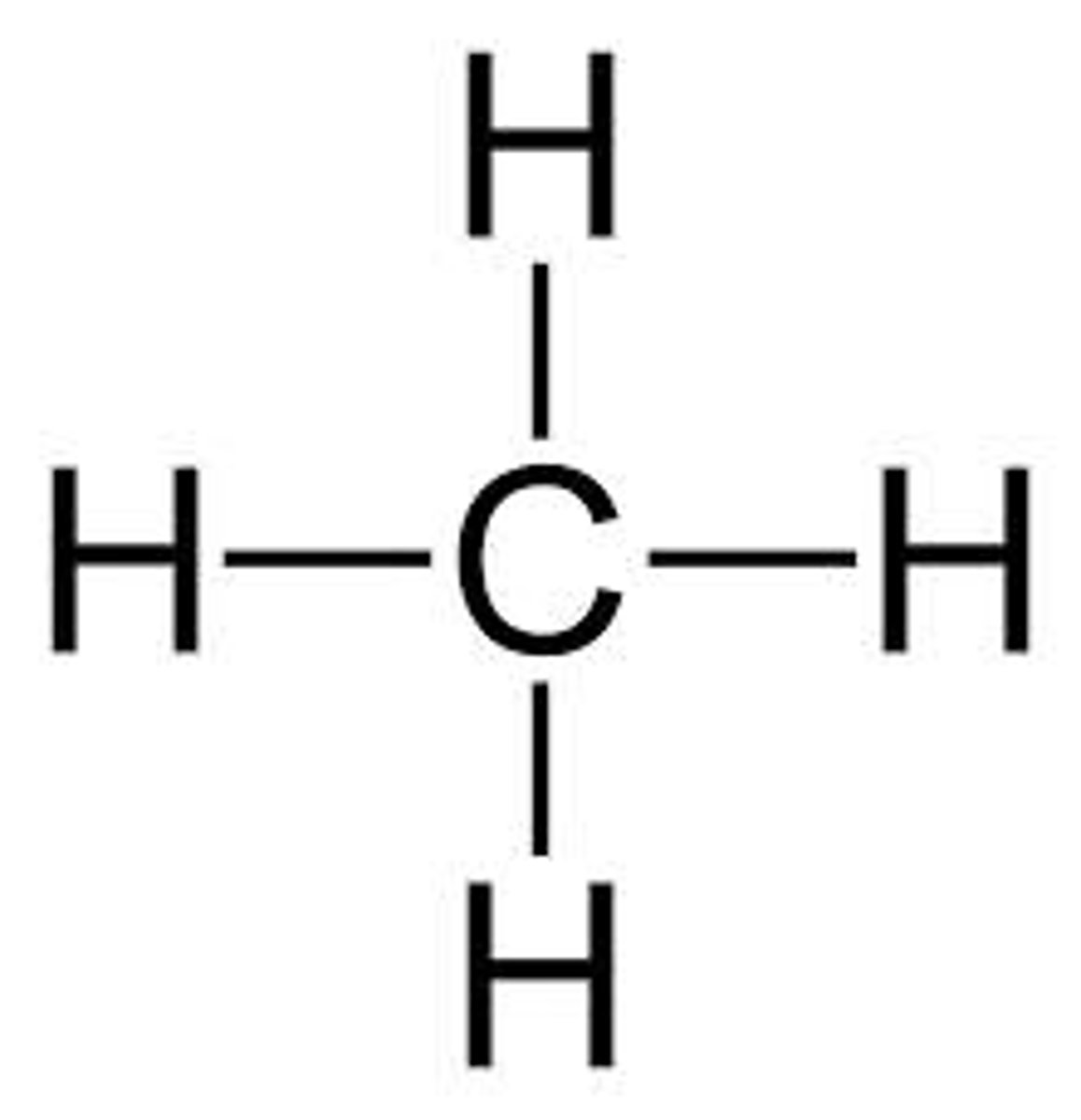
ethane
2 carbon
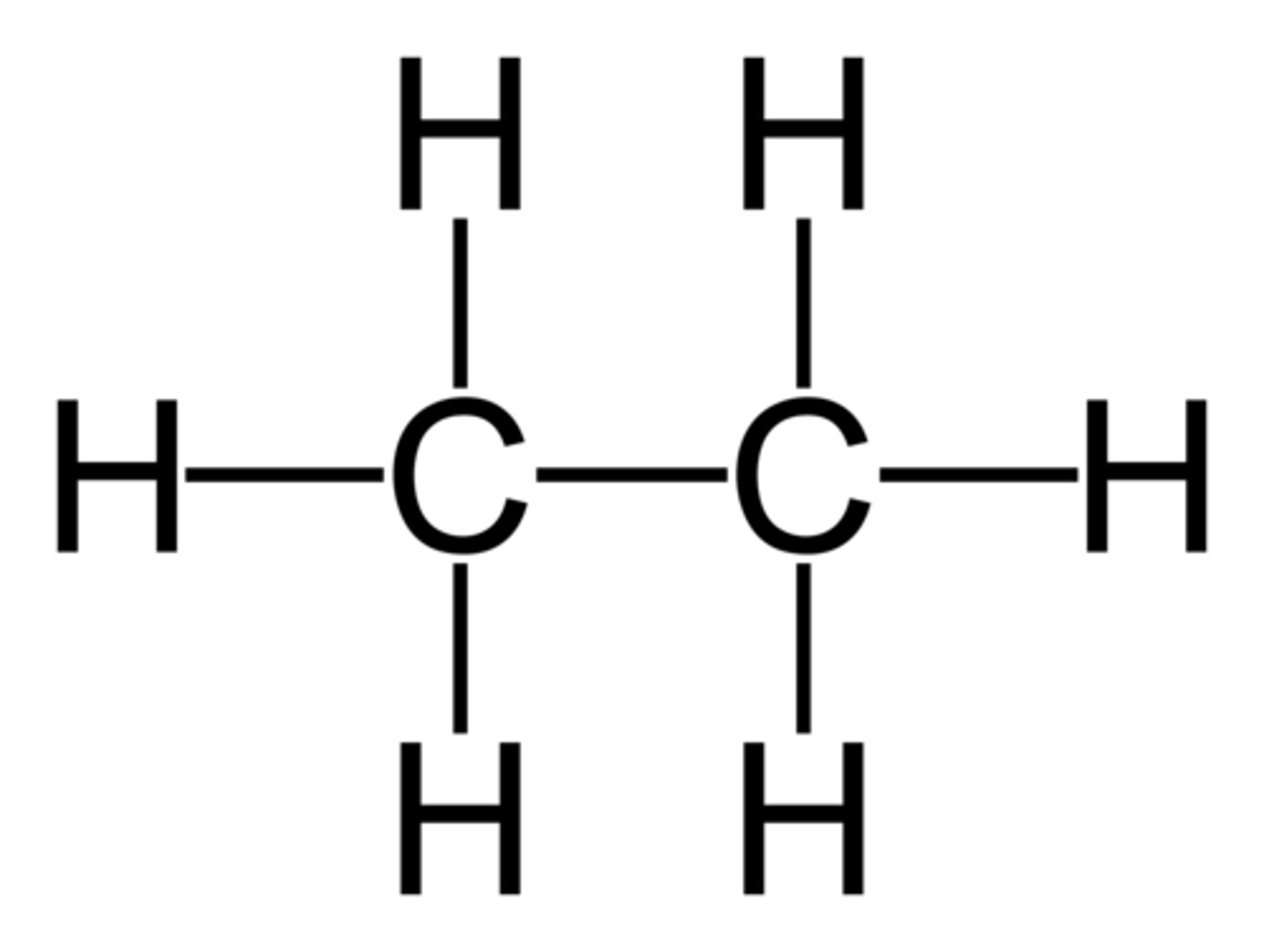
propane
3 carbon
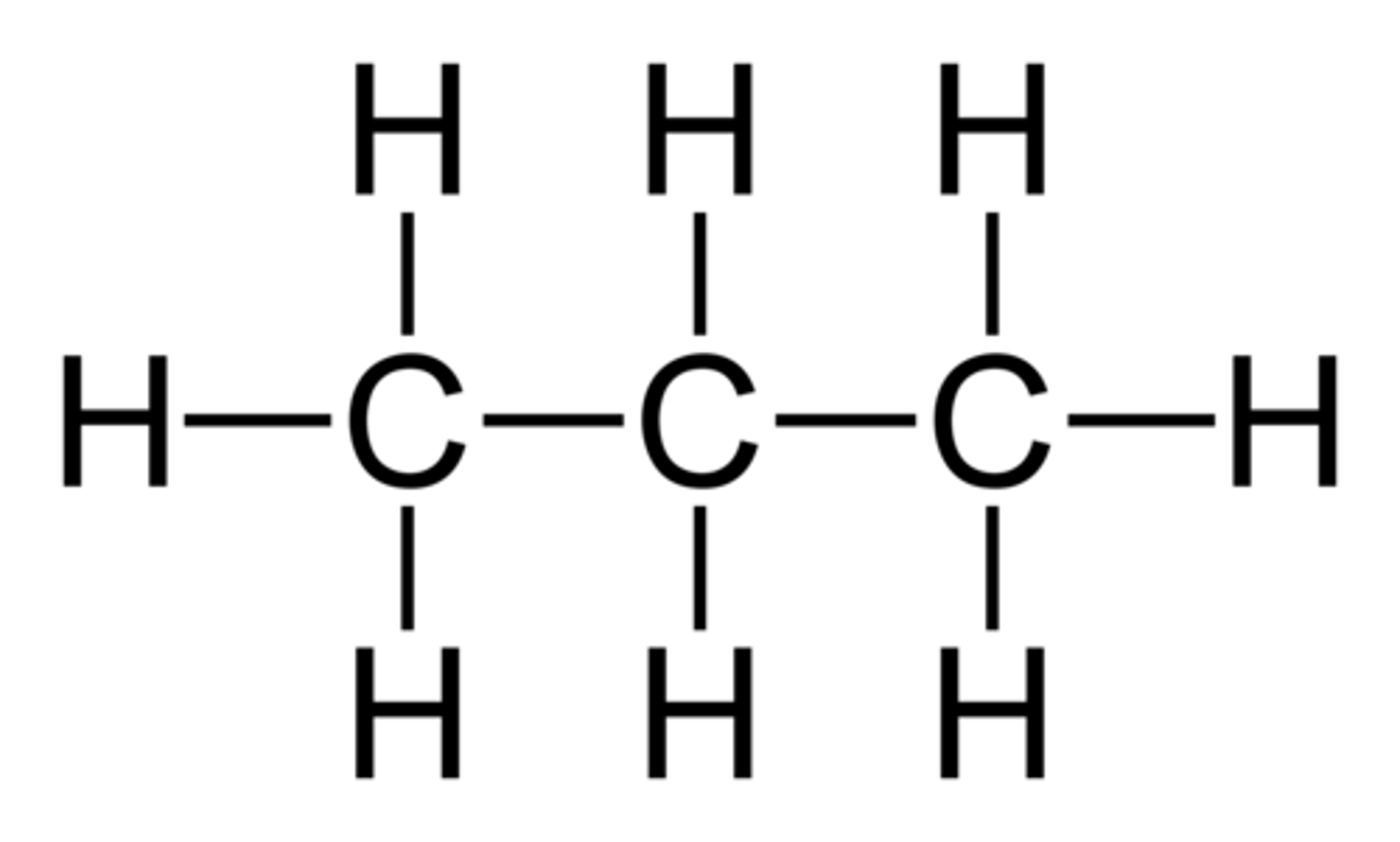
butane
4 carbon
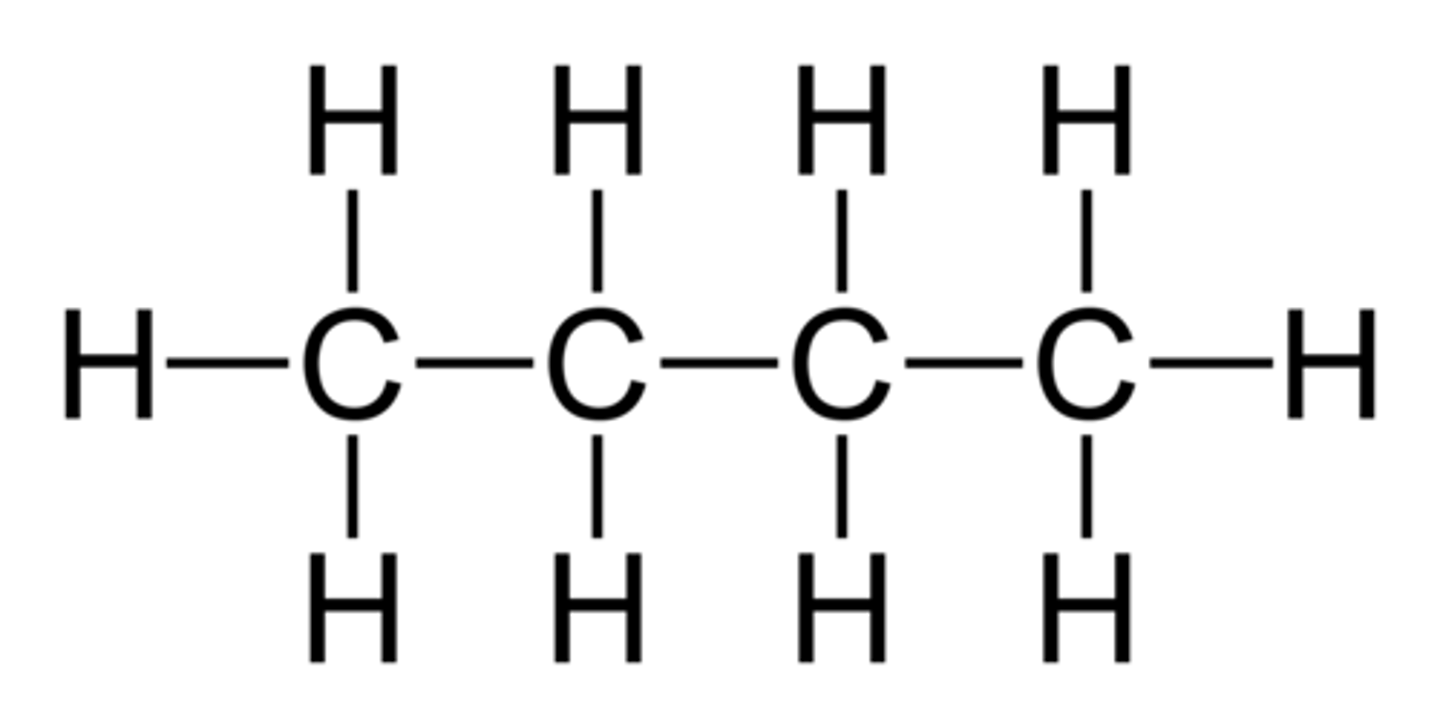
pentane
5 carbon
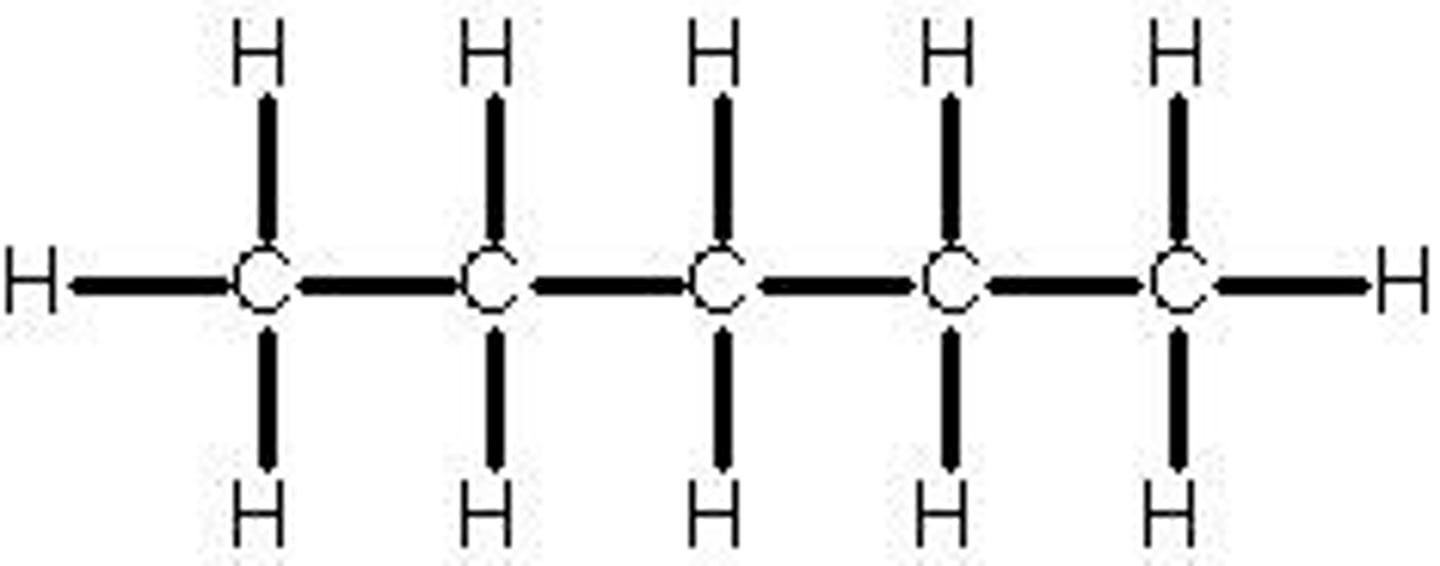
hexane
6 carbon
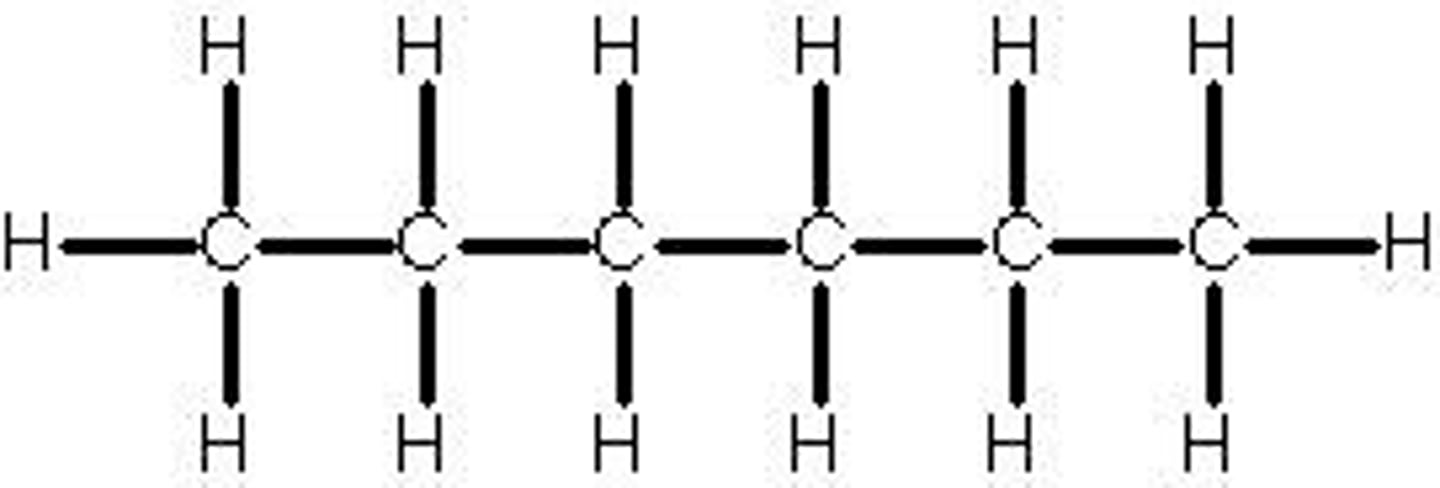
heptane
7 carbon
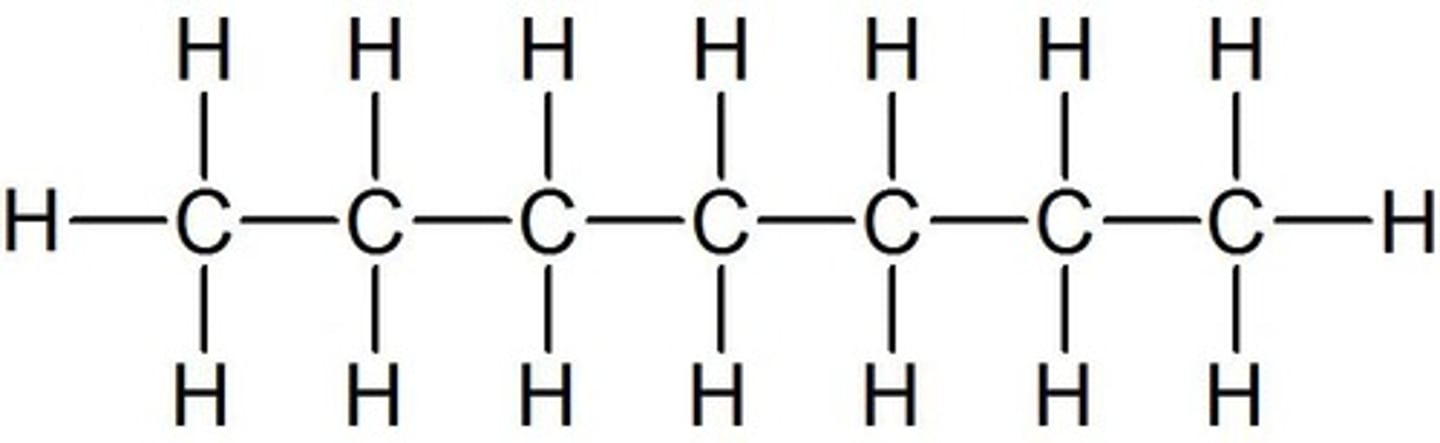
octane
8 carbon
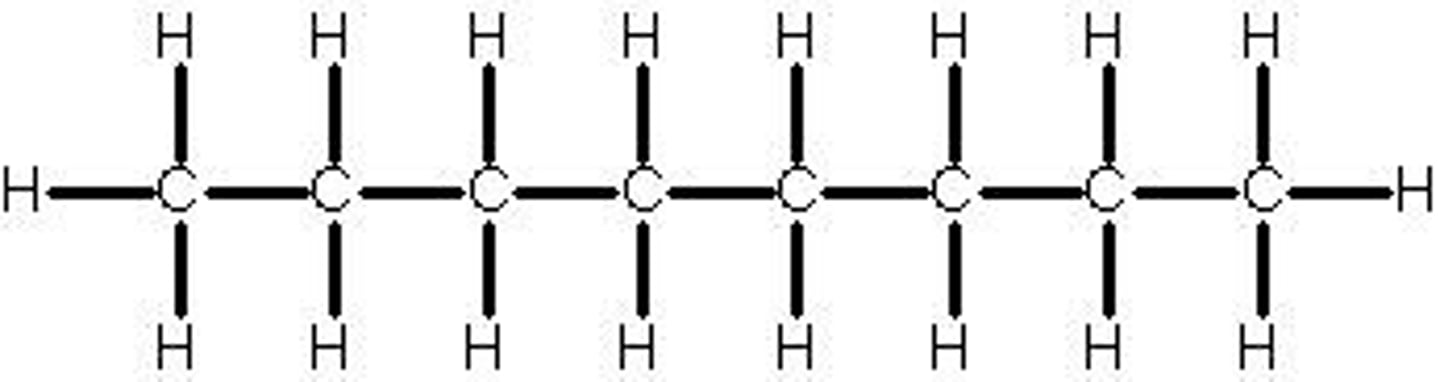
nonane
9 carbon
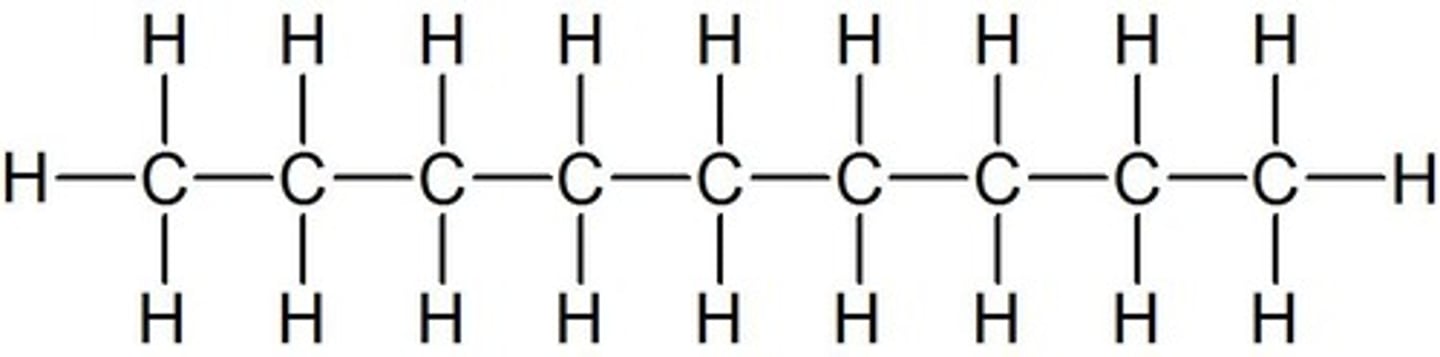
decane
10 carbon
Primary carbon
a carbon bonded to only one other carbon
Secondary Carbon
carbon bonded to two other carbons
Tertiary Carbon
carbon bonded to three other carbons
quarternary carbon
bonded to four other carbons
When do we stop using this naming terminology for carbon?
As soon as carbon is doubly or triply bonded
How are hydrogens charcterized?
To the type of carbon they're bonded to. Ex: Secondary carbon= Secondary Hydrogen
*Quarternary Carbon=0 Hydrogens