2.3. GROUP 17
0.0(0)
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
1
New cards
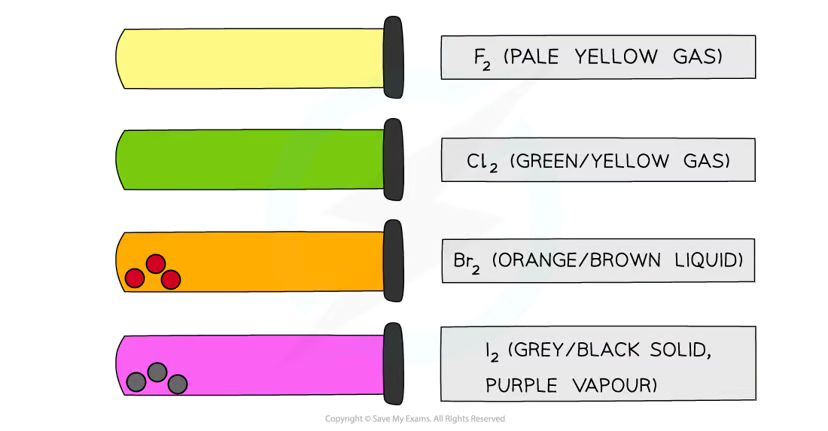
What are the colours and states of chlorine, bromine, and iodine, and what is the trend in colours down Group 17?
Trend:
The colours of halogens become darker as you move down the group due to increasing molecular size and electron density.
2
New cards
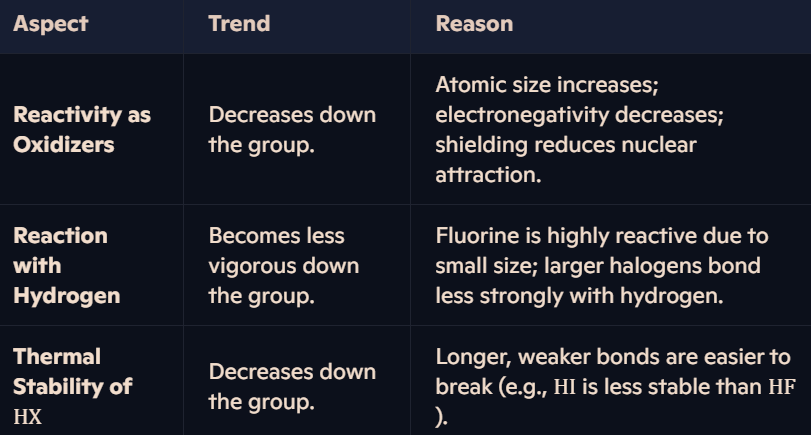
What are the reactions of halogens with hydrogen, and how does reactivity vary?

3
New cards
Halogen Reactivity, Reactions, and Thermal Stabilities
4
New cards
How do halide ions react with aqueous silver ions and ammonia?

5
New cards
What are the reactions of halide ions with concentrated sulfuric acid?

6
New cards
What is the reaction of chlorine with cold and hot alkali? Why is it a disproportionation reaction?

7
New cards
Halide Ions and Chlorine Reactions
