pkas
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
70 Terms
H3O+
-2
HCR3 … CH4 (alkanes)
50
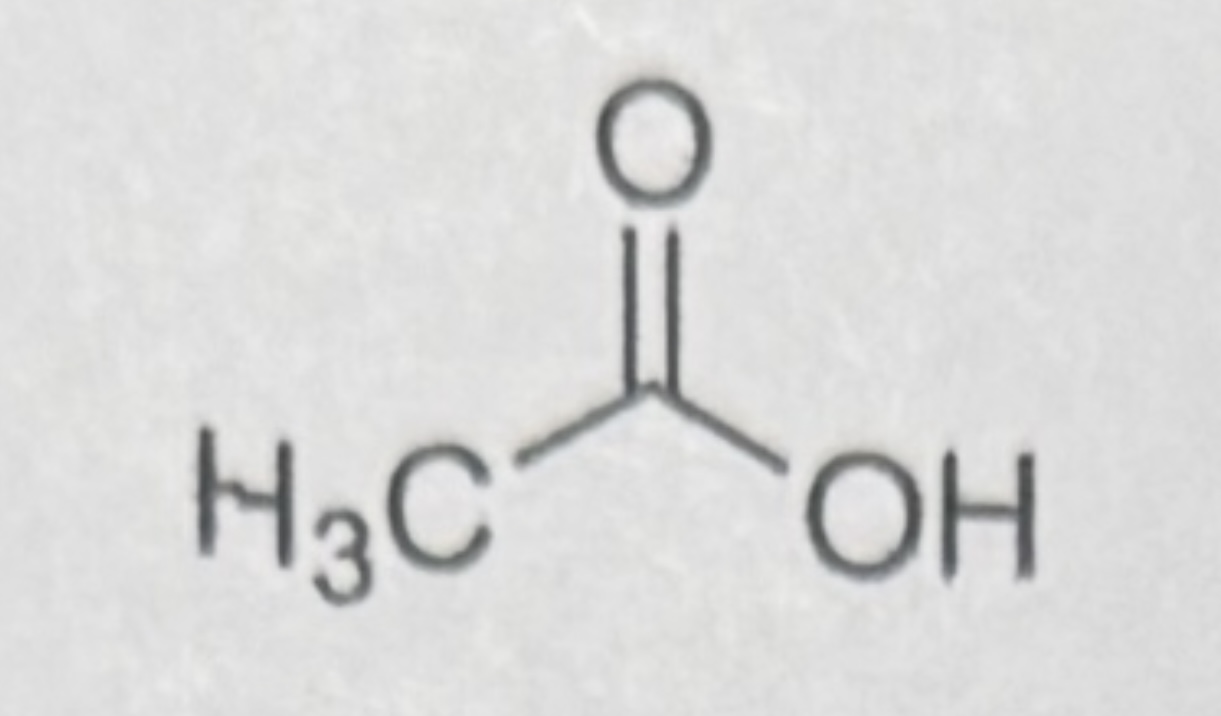
CH3COOH (acetic acid)
5
H3COH (methanol, alcohol)
16
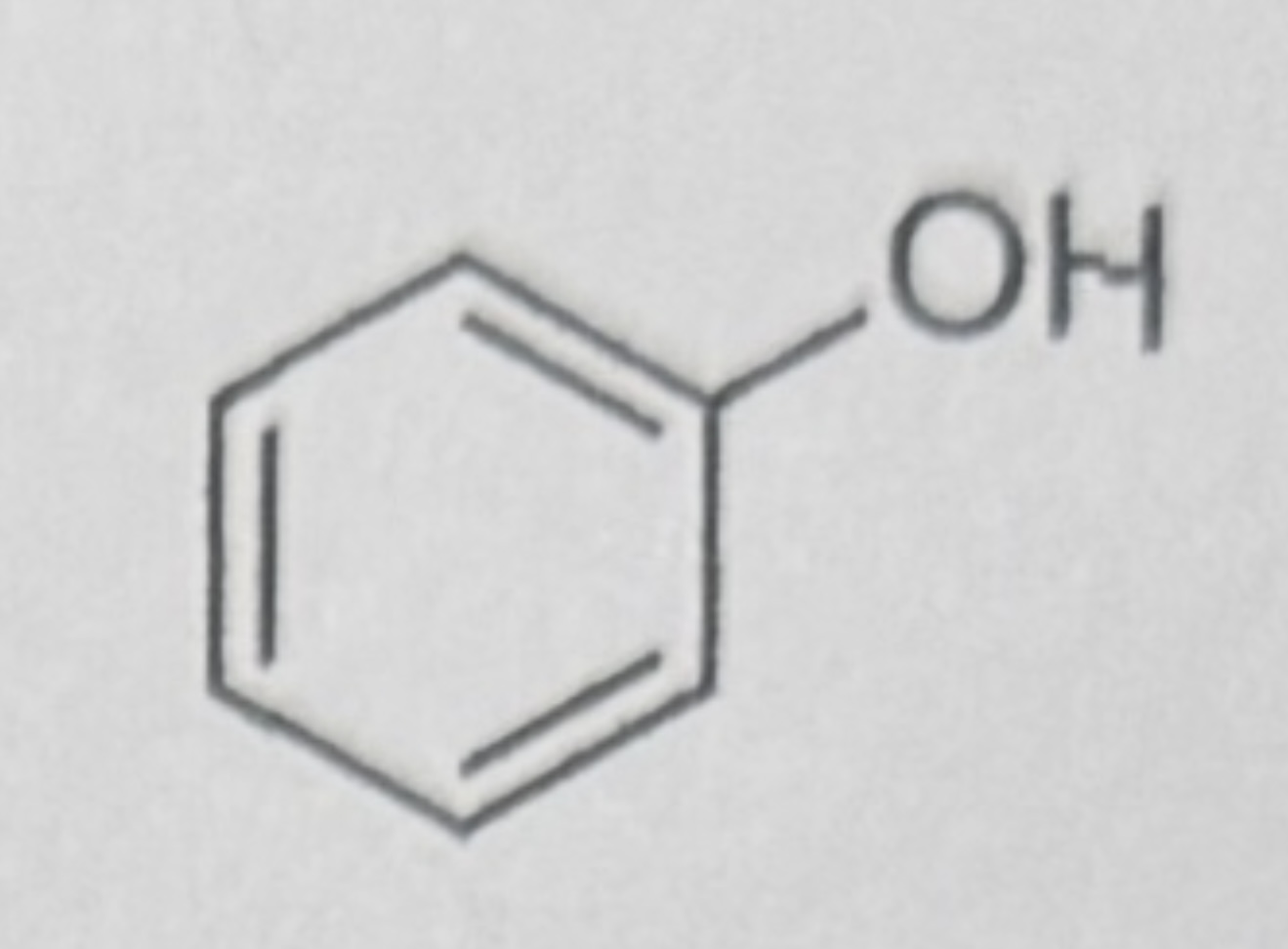
Phenol
10
H2SO4
-5
NH3 (amine)
38
5
HCN
9
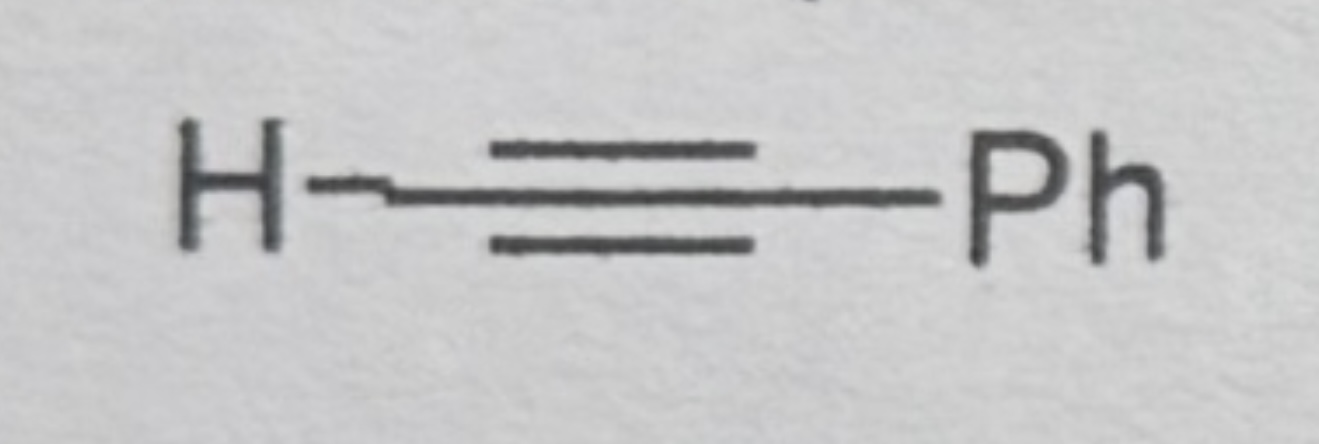
Phenylacetylene
25
H2
40
HCl
-7
HBr
-9
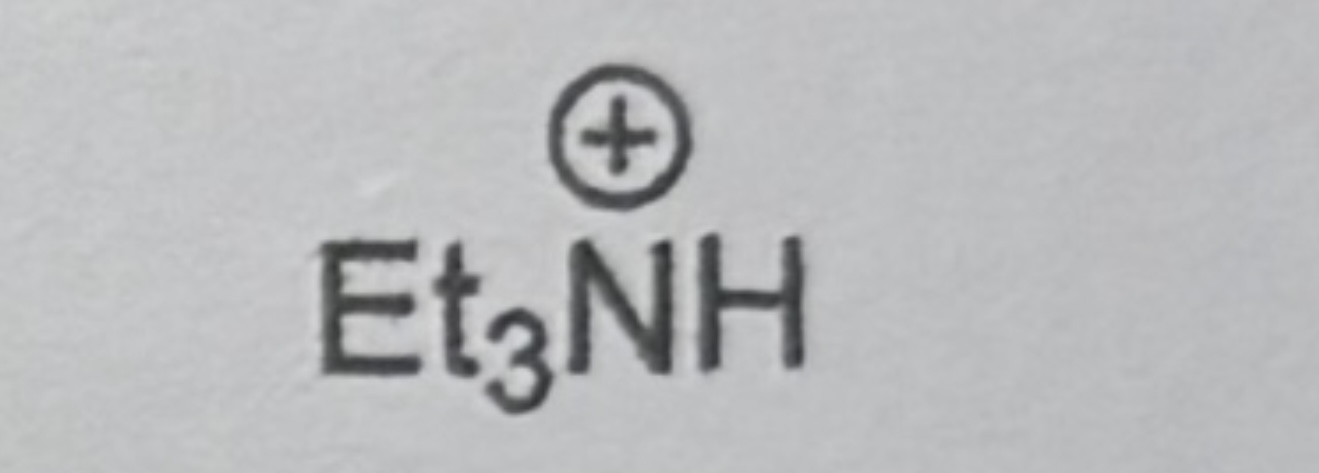
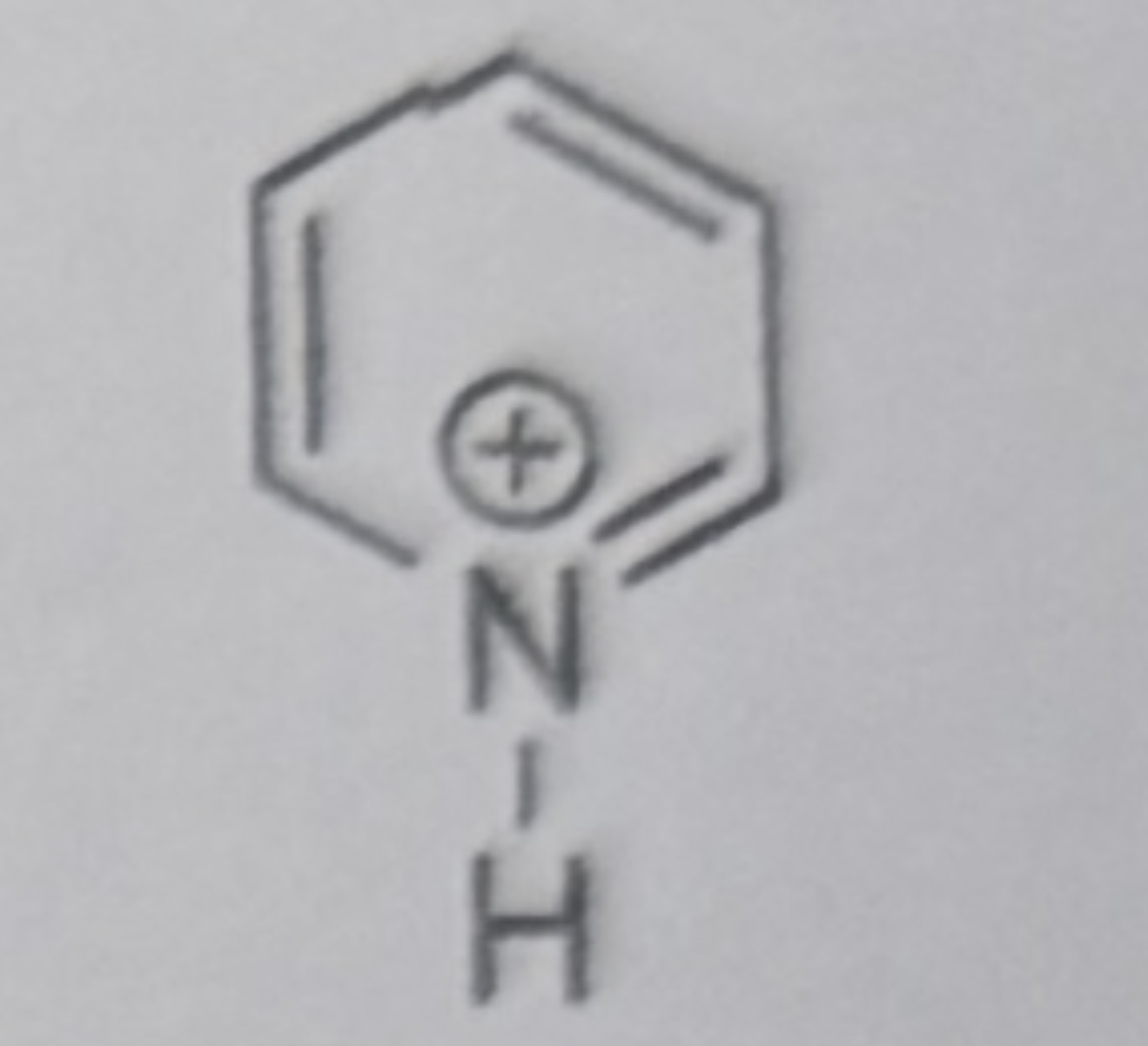
Triethylammonium ion
11
HI
-10
HN3
5
HF
3
HOH/H2O
15
Good LG
EN & polarizable
Low pka value
The TS for an SN2 rxn is
trigonal pyramidal
Williamson Ether Synthesis (SN2)
RO- + alkyl iodide —> ether
SN2 mechanism
Nuc: (usually negative) backside attacks the alpha carbon, which displaces the LG and causes inversion of configuration
SN2 wants
Sterically unhindered E+ (methyl, primary, secondary)
Good LG (EN and polarizable, low pKa)
Very basic Nuc: (high pKa)
Sterically unhindered Nuc: (no tertiary)
SN1, SN2, E1, and E2 require a(n) ___ hybridized E+
sp3
(-) charged Nuc: (usually SN2)
-OH, -OR, CH3CO2-
N3-
-CN HC≡C-
Cl- Br- I-
HS- RS-
Neutral Nuc: (SN1)
H2O ROH
NH3 RNH2
H2S RSH
SN1 mechanism
LG leaves, creating a carbocation
Nuc: attacks the alpha carbon & bonds to it with a positive charge. It can attack from the top or bottom, leading to a racemic mixture
Another Nuc: or LG molecule) use Nuc: if in solution) deprotonates the Nuc: bonded to the alpha carbon
HOCH3
Methanol (good for SN1)
The intermediate carbocation in an SN1 rxn is …
Flat
Carbocation stability
Tertiary = secondary benzylic > secondary allylic > secondary > primary (very rare)
Secondary vs secondary benzylic vs primary
Fastest SN1: secondary benzylic > secondary > primary
Fastest SN2: primary > secondary benzylic > secondary
An SN1 rxn wants
Stable carbocation (secondary and above)
Good LG (low pka)
Okay/neutral Nuc: (Structure doesn’t matter)
KOtBu
Potassium t-butoxide (good for E2)
E2 rxns like ___ bases
strong bases:
K+ -OtBu
Na+ -NH2 (alkynes)
DBU
E2 mechanism
Nuc: deprotonates, C-H bond forms a C=C bond, kicks off LG
E2 requires ____ configuration of deprotonated H & LG
Antiperiplanar (180 degrees from each other in Newman projection)
E2 is always an anti elimination!
Terminal alkene
Alkene at very terminus of alkyl chain
Alkene stability
Tetrasubst > Trisubst > Geminal (1,1) > Trans > Cis > Monosubst >
E2 wants:
Tertiary alkyl halide
Good LG (I > Br > Cl)
Big, strong base (high pka)
Trans
E
Cis
Z
E2 is very selective — draw …
only major product (for disub, trans alkene)
E1 is not as selective — draw …
all products (E, Z, terminal alkene, etc)
We have mixtures of both SN1 and E1 bc
they’re linked & completing in the same flask
E1 Mechanism
LG leaves and forms a carbocation
Nuc: attacks electrophilic C
Nuc: is dep+ated
RDS in E1 and SN1 mech is …
Formation of a carbocation
SN2 likes
Methyl > 1 > 2 alkyl halide
I > Br > Cl LG
Strong base (high pKa)
Small nuc:
SN2 Nuc:
(-) :C≡C-R
(-) O-Et
(-) C≡N
CH3COO(-)
E2 likes
Tertiary
I > Br > Cl
Strong base (high pKa)
Bulky base
SN1 & E1 (together) like
Tertiary E+
I > Br > Cl
Weak base (low pka)
Neutral base
E2 bases
KOtBu (see this —> probably E2)
NaNH2 (2x, used for alkynes)
DBU
SN1 & E1 bases
HOEt
HOH
CH3COOH
HOCH3
Alkyl tosylates behave like
Bromide
Dehydration
Get rid of H2O
E1 Alcohol Reagent
H2SO4 (Acid)
E1 OH Mech
OH on E+ dep+ates H2SO4
H2O leaves, forming a carbocation
LG H2O dep+ates E+ & C-H bond donated its e-s to form a new pi bond
If two CD3 and two CH3 groups are on an alkene, trans and cis are a 1:1 mixture because
their size is identical! CD3 is just slightly heavier than CH3
E2 OH reagent
POCl3 (not acid or else we would form a carbocation)
Pyridine
SN1 OH reagent
HX (X = Cl, Br, I)
SN1 OH Mech
OH on E+ dep+ates Nuc: (H-Cl), pushing e-s onto Cl
H2O group leaves
Cl- from dep+ated Nuc: attacks electrophilic C
Pyridine can/cannot deprotonate an alcohol
cannot!
Pyridium pka = 5
H2O pka = 15
SN2 OH reagent
SOCl2 + pyridine (Cl substitution)
PBr3 + pyridine (Br substitution)
Alkyl tosylate OH reagent
TsCl
Pyridine
Epoxide basic conditions facts
-SN2 to break open ring: use all good SN2 Nuc:s —> -C≡N, -C≡C-R, CH3COO-, -OEt
-Attack less subst C
Epoxide basic conditions reagents
(-) :Nuc
H2O
Epoxide basic conditions mechanism
Nuc: attacks least subst C, breaking C-O bond & pushing e-s onto O. If there are substitutents on the least substituted C, they shift up
(-) O de+ates H2O
Epoxide acidic conditions facts
-Still SN2, but use acids instead
-Attack most substituted C
-Acidic conditions, so all organic media (+) or neutral —> no alkoxide anions!
Epoxide acidic conditions reagents
HOEt / H2SO4 / HBr / HCl etc
Epoxide acidic conditions mechanism
O dep+ates H-Cl, pushing e’s onto Cl
Cl- attacks more substituted C, severing C-O bond and pushing e-s onto O
True or False: H-Cl is NOT H+. H+ doesn’t exist because it’s always ligated by smth in solution!