Exam 3: Dr. Olah Info
1/146
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
147 Terms
Cell injury
Cell is unable (or no longer able) to adapt to a stress, leads to detrimental physical/functional changes
Targets of cell injury
ATP production, cell membrane integrity, cytoskeleton, protein synthesis, DNA replication
cell injury outcomes
may be reversible if stressor is removed, irreversible can result in apoptosis or necrosis
Oxygen paradox
Oxygen is important for live but can be toxic in reduced forms
What is a radical vs free radical and what are characteristics of radicals?
molecule with a single unpaired electron, capable of independent existence; typically generated when a molecule gains an electron, usually oxygen or oxygen containing species (ROS) or nitrogen containing species (RNS), highly reactive
How are free radicals formed?
Natural process, normal reactions in healthy cells (enzymatic or non-enzymatic reactions), can form due to toxin exposure
Cell injury
Cell is unable (or no longer able) to adapt to a stress, leads to detrimental physical/functional changes
Targets of cell injury
ATP production, cell membrane integrity, cytoskeleton, protein synthesis, DNA replication
Superoxide radical
forms when molecular oxygen picks up another electron (3 total), some oxidant activity with limited membrane permeability
Source of intracellular ROS
ETC transports electrons, sometimes leak from a protein and are picked up by oxygen to form a superoxide radical
Generation of hydrogen peroxide and hydroxyl radical
a superoxide radical is converted to hydrogen peroxide via superoxide dismutase, easily generates the hydroxyl radical (extremely reactive and has limited ability to cross membranes)
Steps of hydroxyl radical generation from hydrogen peroxide
H2O2 may be converted to OH via the Fenton reaction, non enzymatic but requires iron or copper as electron donor
H202 may also react with a superoxide radical in the Haber-Weiss reaction (non-enzymatic)
Cytochrome P450 enzymes
heme-containing enzymes, metabolism of endogenous compounds, use electrons to activate oxygen for reaction, has “leaky” points (ROS)
NADPH Oxidase
important in cells of immune system, activated as response to invading pathogens, uses oxygen to generate free radicals to kill organism in conjunction with phagocytosis (respiratory/oxidative burst)
Xanthine oxidase
necessary for final steps of purine catabolism to uric acid, molecular oxygen is reduced to a superoxide radical
Monoamine oxidase (MAO)
metabolism or catecholamines in nerve terminals and other cells, generates H202
What environmental factors can increase ROS?
ionizing radiation (ultraviolet, x-rays), pollutants, cig. smoke, chemicals, drugs
Nitric oxide
free radial, important in widespread signaling molecule, vasodilator, neurotransmitter, usually at low concentrations that don’t cause damage
Protection from ROS
antioxidant enzymes, non-enzymatic antioxidants, repair processes, sequestration, drugs/supplements
Superoxide dismutase (SOD)
primary defense against oxidative stress, three isoforms that differ in location and regulation of expression but all use a metal ion in catalysis
Mn SOD
expressed in mitochondria, critical enzyme, levels may be increase by stress, expression is altered in various cancers
CuZn SOD
expressed in cytosol, Cu used in catalysis, Zn used in enzyme stability, not highly susceptible to regulation
Glutathione peroxidase
contains selenium, located in mitochondria and cytosol, handles H202 outside of peroxisome
Glutathione reductase
necessary to cycle glutathione disulfide (GSSG) back to reduced form (GSH)
Catalase
located in peroxisomes, widely distributed (liver/kidney), involved in beta-oxidation of long fatty acids, H2O2 production, and substrate metabolism
Nrf2
normally bound in cytoplasm by Keap1 (promotes Nrf2 destruction), released by Keap1 in response to oxidative stress
Curcumin
polyphenolic compound in turmeric, activates Nrf2
Vitamin e (alpha-tocopherol)
fat soluble vitamin, widely distributed throughout body (obtained via diet), mixture of tocopherols
lipid per-oxidation chain breaker
allows Vitamin E to donate an electron and stabilize its free radical intermediate
Vitamin C (Ascorbic acid)
regeneration of active form of vitamin E, may interact with free radicals directly (donates electrons)
Flavonoids
multiple compounds with a flavone backbone, may have multiple mechanisms (produce free radicals, free radical scavengers, inhibit Fenton reaction)
Lycopenes
linear unsaturated hydrocarbon, red pigment of plants
Antioxidant effects
quench free radicals, increase expression of antioxidant enzymes
Free radical induced damage
chain reactions are set off and extract electrons from other molecules (DNA, lipids, proteins)
Examples of DNA damage
Mispairing, strand breaks, excision of bases, crosslinking
Guanine and OH mediated modification
frequent site of OH attack where the 8-position of ring is hydroxylated, 8-OH-G is mutagenic and carcinogenic (can be measured in urine and used as a biomarker for oxidative stress)
Results of ROS-induced DNA damage
DNA damage detected, signaling proteins relay message, effector proteins/processes respond, DNA is repaired
Steps of base excision repair
Glycosylase excises the damaged base
Nuclease cuts the phospholipid backbone just before the missing base
Polymerase and ligase adds in a new base
What happens if DNA repair mechanisms fail?
cell replication may stop, cell may undergo apoptosis
Lipid Damage
site of attack is the lipids of membrane; mainly polyunsaturated fatty acids (allows for the free radical to extract H+), occurs in a chain reaction process (lipid peroxidation)
Steps of Lipid Peroxidation
Initiation- lipid becomes a lipid radical
Propagation- lipid radical reacts with oxygen to form lipid peroxyl radical
Degradation- Occurs when cells are damaged, produces malondialdehyde (carcinogenic, found in blood/urine)
Termination- Antioxidant may donate electron or radicals may recombine
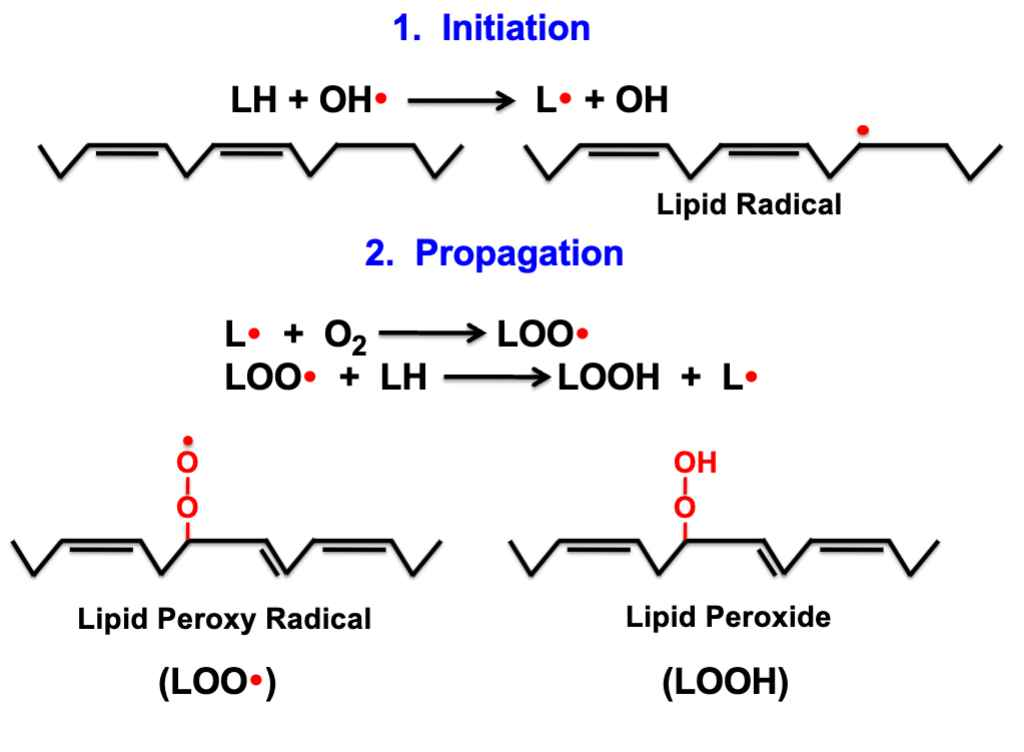
End results of lipid peroxidation
cell injury due to destruction of membranes, other cell constituents affected (cross-links proteins)
Free radical-induced protein damage
free radical extracts H atom from amino acid residue, may target protein backbone or amino acid chain, usually cysteine and methionine
effects of protein oxidation
structure/location dictate whether or not the protein is targeted, cleavage of protein/chemical modification affects function
Types of defense for protein oxidation
Prevention- antioxidant enzymes
Specific defenses- enzymatic system may reverse oxidation of methionine
Degradation of modified proteins by the proteasome- hydrophobic patches of the protein may be exposed and targeted for destruction
Ubiquitin-Proteasome System (UPS) function
Many proteins must be degraded by the cell, either normal protein turnover or a defense mechanism, often very protective, dysfunction can result in cell death or cancer
components of UPS
target protein (ubiquitin), E1/E2/E3 proteins(places ubiquitin proteins on substrate protein), proteasome(complex with regulatory regions can contains proteases in central region)
Cardinal signs of parkinsons (TRAP)
tremor-often observed at rest, “pill-rolling”
rigidity-increased muscle tone
akinesia-slowness, fatigue, interruption in movement
posture- stooped, loss of balance
pathology of parkinsons
loss of dopaminergic neurons in substantia nigra
lewy bodies- intraneuronal structures that lack membranes
sources of oxidative stress
aging, decreased MTC activity, dopamine metabolism
alpha-synuclein
major component of lewy bodies in insoluble fibrillar form, may have role in dopamine synthesis and/or vesicular transport
Benefits of ROS
immune defense-kills microorganisms
inflammation-signaling molecules to increase permeability and leukocyte migration
MAPKs activation-may promote proliferation and/or survival
Supply vs demand in adequate oxygenation
supply- tissue perfusion with oxygenated blood
demand- 90% of O2 used for oxidative phosphorylation in MTC
hypoixa
inadequate oxygenation to meet demands of tissue, usually results from decreases supply
ischemia
overall decreased blood flow to tissue, includes glucose and other nutrients but tissues are likely hypoxic
acute decreased O2 supply
high altitudes, CO poisoning, surgery, pulmonary (asthma attack), pathophysiologic thrombus (MI)
chronic decreased O2 supply
vascular disorders, pulmonary (COPD), heart failure, anemia
compensatory responses to hypoxia
increased respiratory rate (short term), increased synthesis of erythropoietin (long term)
Heterodimeric transcription factors
HIF-1 beta- stable in all O2 conditions
HIF-1 alpha- regulated by O2 levels
vasculogenesis
development of new vessels from precursor cells
angiogenesis
sprouting of new vessels from pre-existing vasculature, typically occurs at the capillary level, involves endothelial cells and sometimes pericytes if forming more fully developed vessels
schematic of angiogenesis
pericytes and extracellular matrix (ECM) are loosened
endothelial cell migration
endothelial cell proliferation
Role of matrix metalloproteinases
degrades the basement membrane of endothelial cells via proteolytic activity (enzymes that break down cells)
Process of endothelial cell migration
cells respond to attractive/repulsive signaling cues (involves receptor activation of small G proteins)
Process of endothelial cell proliferation
EC number must increase to make more vessels, enhanced by angiogenesis, slow turnover rate
Process of endothelial cell tube formation
represent proliferation and migration but also morphological changes that allow for branching and lumen formation
process of endothelial cell survival
proliferating/migrating ECs need protection during these processes, growth factors and their receptors are often anti-apoptotic
Angiogenic stimuli
hypoxia-most potent
receptor tyrosine kinases- growth factors receptors, VEGF
GPCRs-several involved
Vascular endothelial growth factor (VEGF)
secreted protein produced by many cell types, expressions/secretion stimulated by many factors, potent stimulus involved in angiogenesis (stimulates EC reponses)
VEGF receptors
all are RTKs, mostly associated with angiogenic responses on endothelial cells
Additional stages of blood vessel development
blood vessels need to be stabilized by surrounding pericytes
Diseases characterized by impaired blood flow (ischemia)
coronary/peripheral artery disease, diabetes
Limitations of angiogenesis therapies
incomplete understanding, multiple factors needed, difficult to deliver factors
Angiogenesis-dependent disease (progression of the disease)
ocular diseases, cancer, chronic inflammation
angiogenesis and cancer
tumors require vasculature b/c environment is often hypoxic so VEGF is released to promote vascular development (angiogenesis)
Targeting VEGF as a cancer treatment
inhibition of angiogenesis, combination therapy with anti-cancer drugs
VEGF-targeted anticancer drugs
Bevacizumab (avastin) and Sorafenib (Nexavar)
Inflammation
body’s response to injury, involves vascular/cellular responses to defend or repair
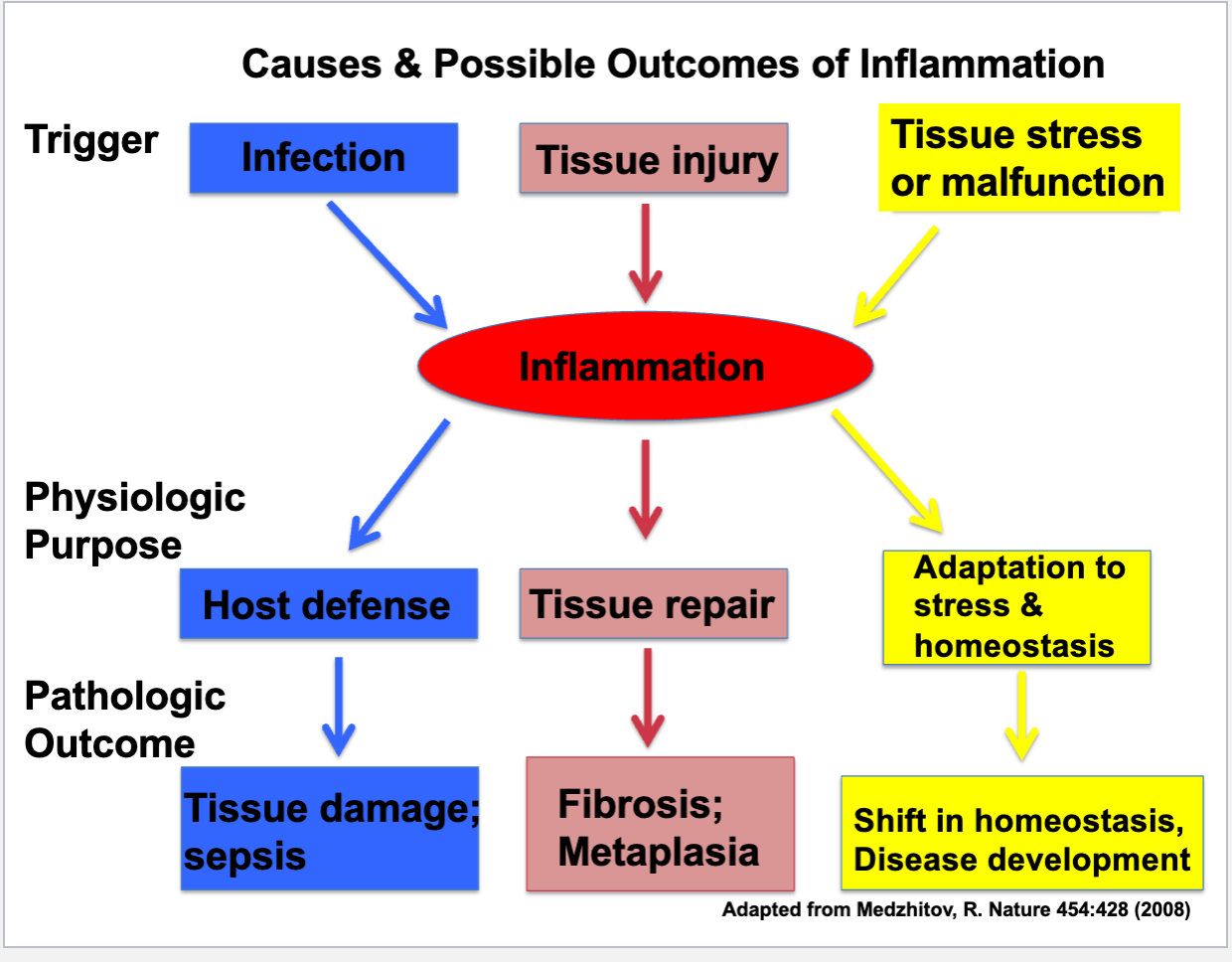
Causes of inflammation
infectious pathogen, foreign bodies, trauma, physical/chemical agents, tissue necrosis, hypersensitivity reactions
Influence of initiating stimulus on inflammatory response
intensity, duration, outcomes of response
examples of bad inflammation
‘itis (asthma, colitis, rheumatoid arthritis)
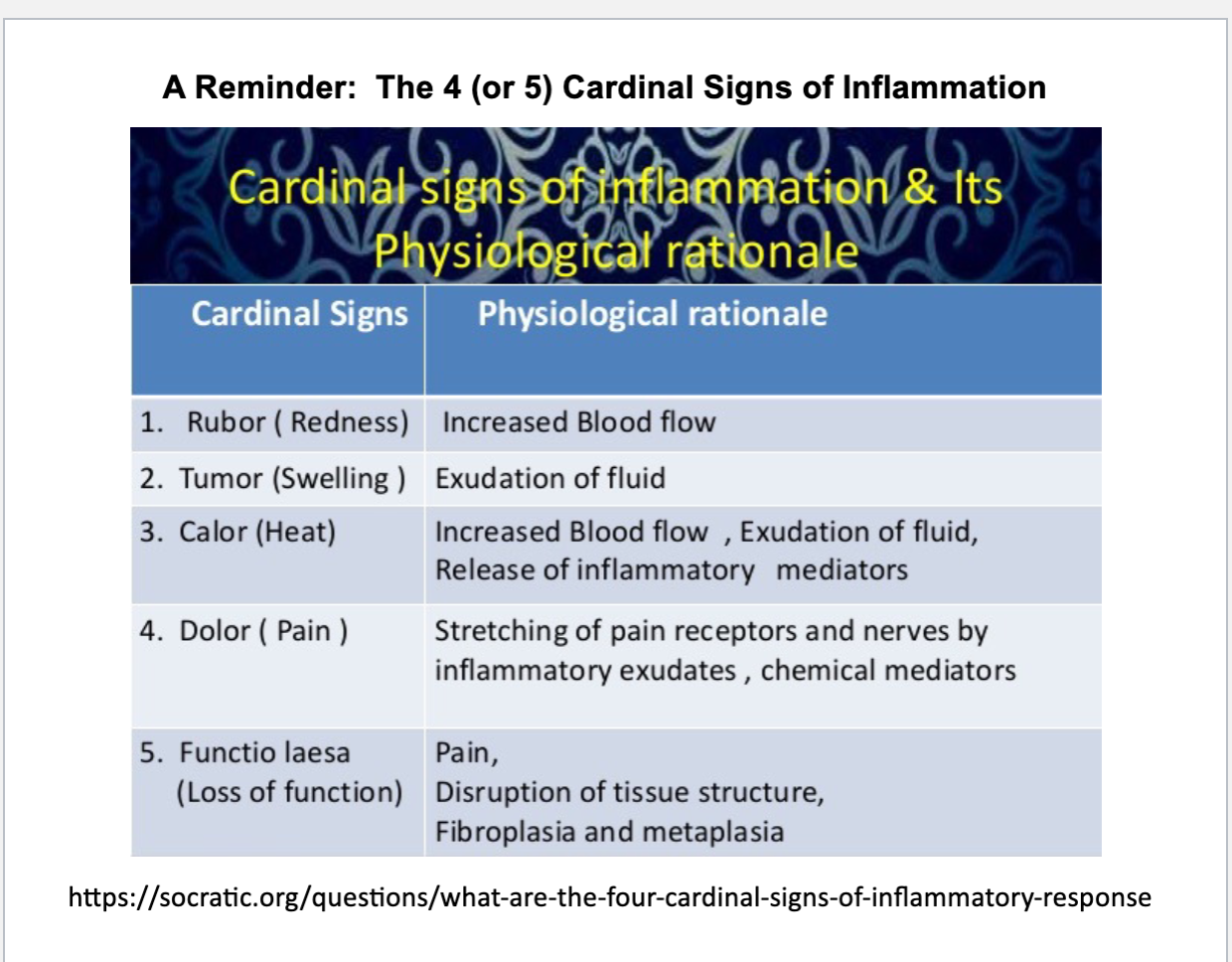
cardinal signs of inflammation
rubor (redness), swelling, calor (heat), dolor (pain), functio lasea (loss of function)
cytokines
proteins or peptides released by immune cells, activate cell surface receptors, involved in inflammatory process and cell signaling
vasodilation
increases blood flow to an area, result in calor and rubor, primarily observed in arterioles ex: histamine
paracellular leakage
junctions between ECs become loosened due to contraction of individual ECs
transcellular leakage
limited transport of proteins through ECs
causes of increased vascular permeability
EC injury (burn/frostbite), leukocyte mediated cell injury (infection), angiogenesis (chronic inflammation)
Extravasation of leukocytes
margination
tethering & rolling
integrin activation
firm adhesion
transmigration (diapedesis)
migration in interstitial tissue (chemotaxis)
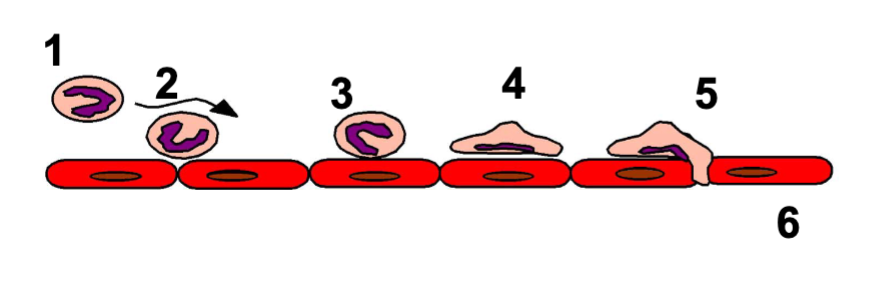
margination
leukocytes accumulate on surface of blood vessel, occurs due to low blood flow
tethering & rolling
marginated leukocytes interact with endothelial cells and slow down, mediated by selectins (adhesion molecules)
types/structure of selectins
E-selectin: endothelial cells
P-selectin: platelets & endothelial cells
only expressed at surface of ECs that have been activated by cytokines or other inflammatory mediators
selectin-ligand interaction
ligands provide a region for proteins to bind, serves as the “tether”, fast on & fast of kinetics (rolling response)
integrins
located on leukocytes, involved in cell-cell or cell-matrix interactions, heterodimeric (alpha and beta domain)
Integrin activation
necessary for firm adhesion of leukocytes, smooth/round cell becomes flattened with extensions, use inside-out signaling and works with chemokines
Inside-out signaling
signaling comes from inside the cell and initiates a response outside the cell
Types of integrin receptor ligands on endothelial cells
Intercellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM)
How is surface expression of ICAM and VCAM upregulated?
by inflammatory cytokines and other signals, high affinity results in firm adhesion of leukocytes to endothelium
Outside-in signaling
used by ICAM and VCAM when integrin binds
Leukocyte transmigration (diapedesis)
leukocytes must leave the blood vessel(venule) to get to the damaged tissue without damaging the venule, usually paracellular(between adjacent ECs)
Paracellular transmigration
initiated as EC extends around adherent leukocyte, leukocytes may form pseudopods necessary for migration (same components as firm adhesion)