Tertiary and Quaternary Structure
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
what is tertiary structure
its the 3D structure taking into account interactions between back bones and R groups of amino acids
determined by primary structure
what is Quaternary Structure
Its the spatial arrangement of each of a proteins sub-units
Sub units are different polypeptide chains
Bonds that stabilize T and Q structure
1) Disulphide bonds → between cysteine residues, common on extracellular proteins
2) Ionic interactions / salt bridges → between oppositely charged amino acids
3) Hydrogen bonds → between side chains, between peptide groups , between side chain and peptide group and with water
4) Hydrophobic interactions and VDW forces
listed form strongest to weakest
The hydrophobic effect
helps proteins get into there TS
Hydrophobic effect helps limit non polor and hydrophobic molecules intercation with water
this is because the increase in entropy of water would have formed cages aroudn the molecule
the hydrophobic effect causes chain that has NP hydrophobic side cahins to fold and end up on inside of proetin
and side chain that are polar and hydrophilic end up on the outside
Hydration shell
water can interact with polar hydrophilic side chains through H bonds to stabilize protein → called hydration shell
water can also directly interact with backbone and side chain on the interior protein though buried water molecules
what other compounds can stabilize TS and give an example
metal ions and ligands
e.g. Zn fingers
How do Zn fingers work
they are nucleic acid binding proetins
finger like strucure includes alpha helix and 2 short antiparalle B sheets held togetehr by Zn
stabilized by a Zn ion that is tetrahedrally coordinated using CYs,HIs and Asp or Glu side chains
Zn fingers too small to be stable without Zn
what data base allows for proetin structure to be analysed
Proetin data bank (PDB)
what has been described to be within protein folds
super secondary structure or motifs
are an intermediate level of organisation between secondary structure and tertiary structure
Examples of motifs
β-α-β motifs
Greek keys
α-α hairpins
Zn finger motifs (same as before)
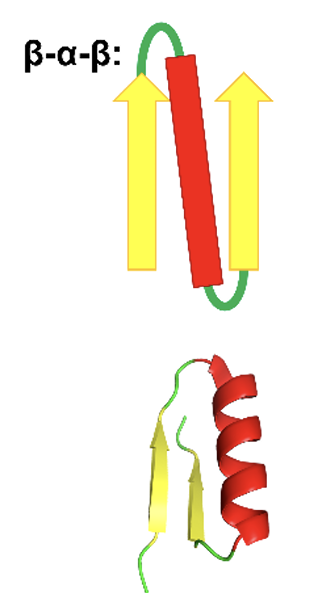
β-α-β motifs
antiparalle B - sheets are joined by short terns
parallel B -sheets are joined together with longer turns usually with an alpha helicase segment
hydrophobic residues interact between alpha and b- sheets
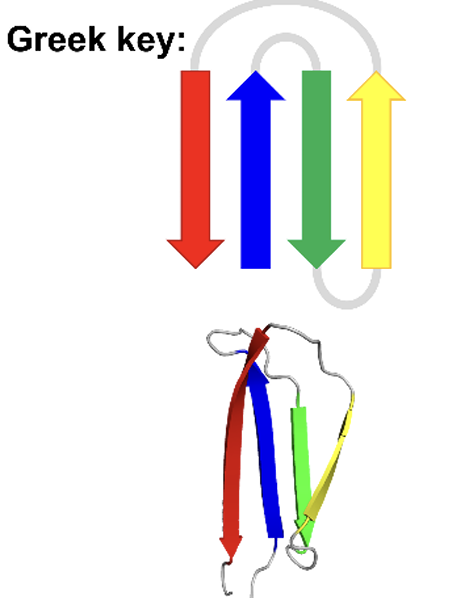
Greek motif
larger motif
when have consecutive 3 anitparallel B-sheets connected by small hairpins followed by a larger connection to the 4 B-sheets which lies adjacent to the first
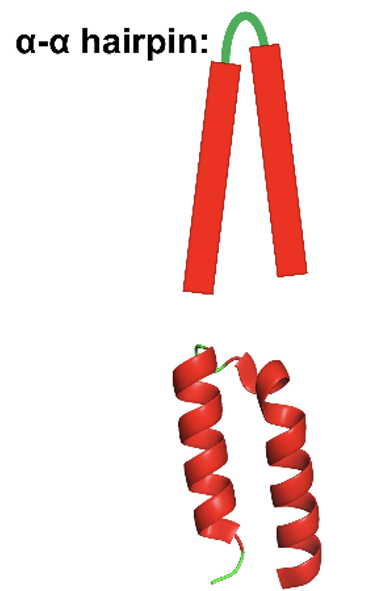
α-α hairpins
the connection between 2 antiparallel a helicase
the longer the loop the greater the number of combinations
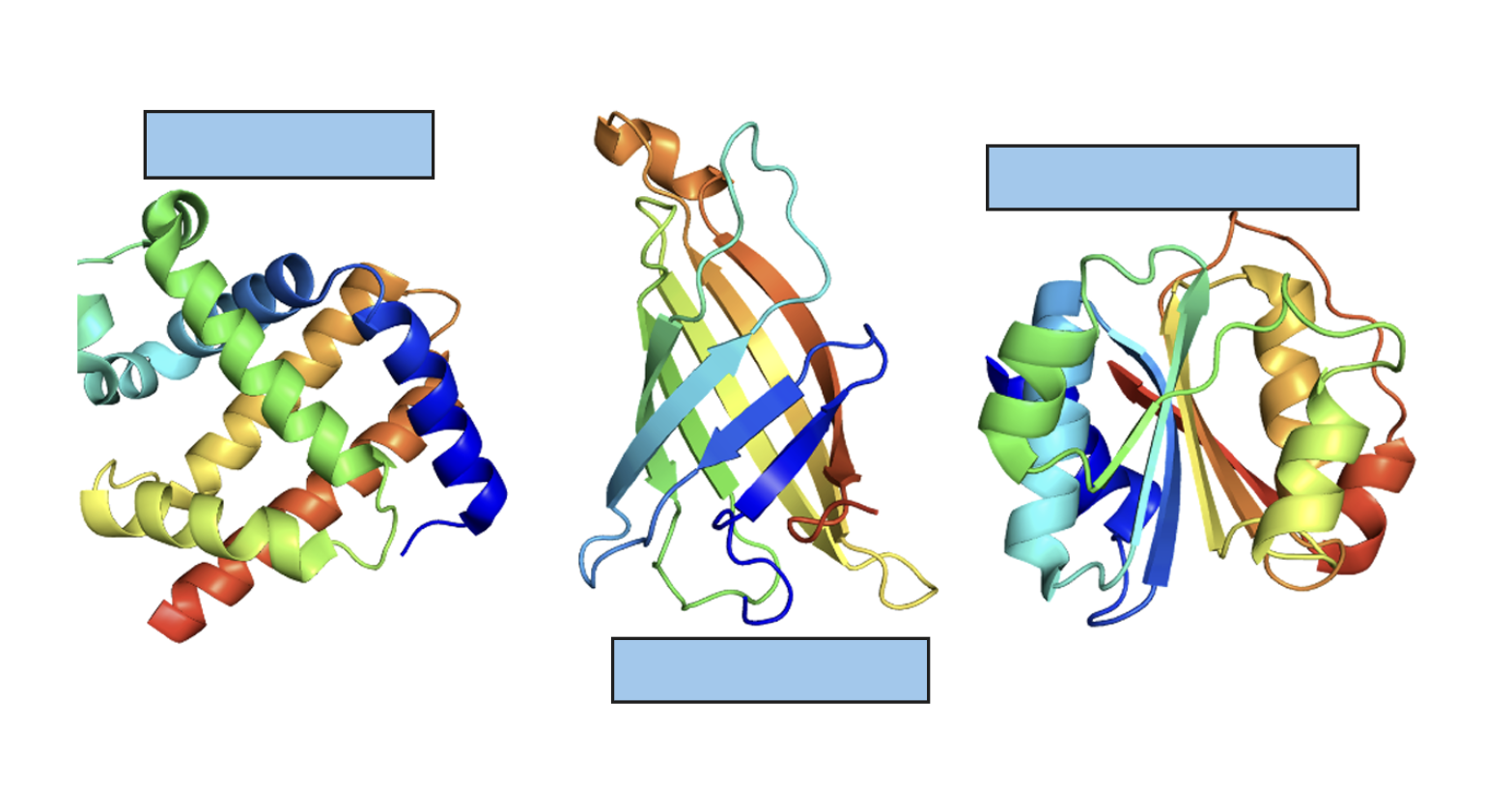
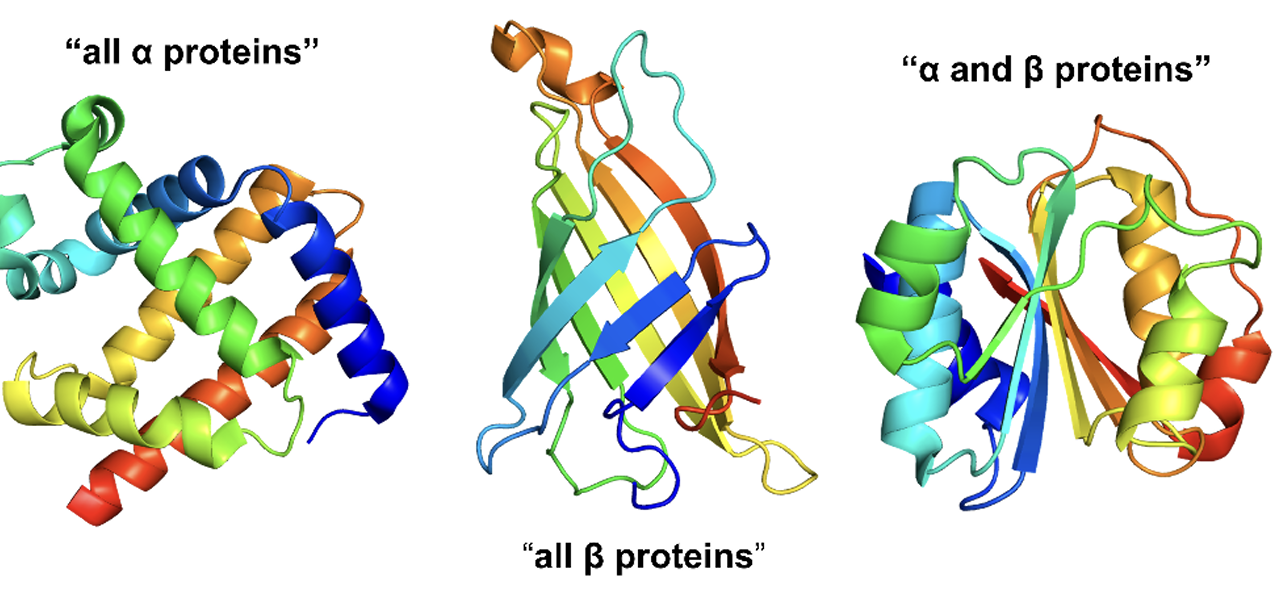
Domains in pyruvate kinase
has 3 domains:
a β-sheet domain
two are of the α/β type
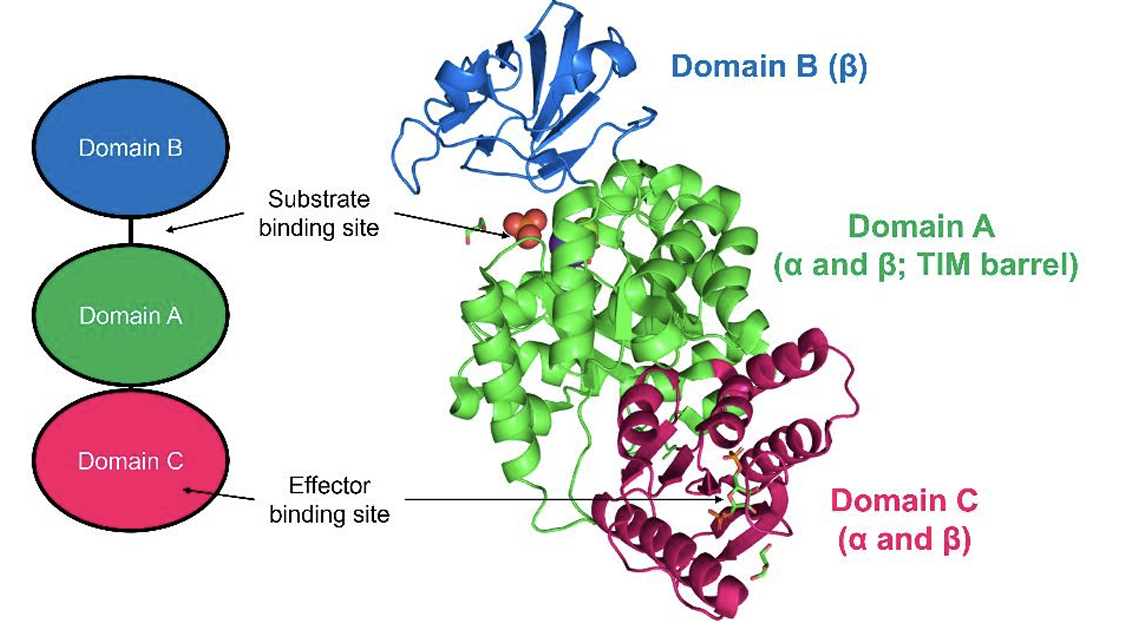
Quaternary structure describes a protein composed of two or more polypeptides, called
subunits
what are homodimers
multiple of the same sub unit
what are hetrodimers
dimers made up of different sub-units
Collagen
Fibrous protein - structural role

Hemoglobin
Is a Globular proteins - functional role

insulin receptor
type of membrane protein

what are protein dynamics
the movements and conformational changes that proteins undergo to perform their functions
covers a large range of movement amplitudes and timescales
Protein dynamics can be versatile and covers a large range of movement amplitudes and timescale
Sub-angstrom vibrations of covalent bonds represent the fastest of those movements (fs)
Then come side-chain rotations (ps–ns)
and backbone fluctuations (ns),
followed by loop motions (ns–ms),
ligand binding/unbinding events (>100 ns),
and collective domain movement (>µs).
loop motions
when the substrate binds the triosephosphate isomerase enzyme, a loop changes from an ‘open’ conformation (green) to a ‘closed’ one (red), which prevents solvent access to the active site and stabilises the intermediate compound of the reaction
Domain motions
For example, bacterial malonyl coenzyme A (CoA) synthetase undergoes a conformational change when the substrate binds: the C terminal lobe rotates upon ATP / Mg2+ binding
what are Intrinsically Disordered Proteins (IDPs)
proteins that are partially or completely unstructured
IDPs are malleable, adapting to structurally different partners, making them ideal “hub proteins”! IDPs are also thought to be involved many human diseases, including cancer, Alzheimer’s, and Parkinson’s disease
Receptor and ligand associate via
non covalent interactions
such as hydrogen bonding, electrostatic interactions, hydrophobic and Van der Waals forces, and shape complementarity
what does binding affinity describe
strength of binding between receptor and ligand
what is it measured in
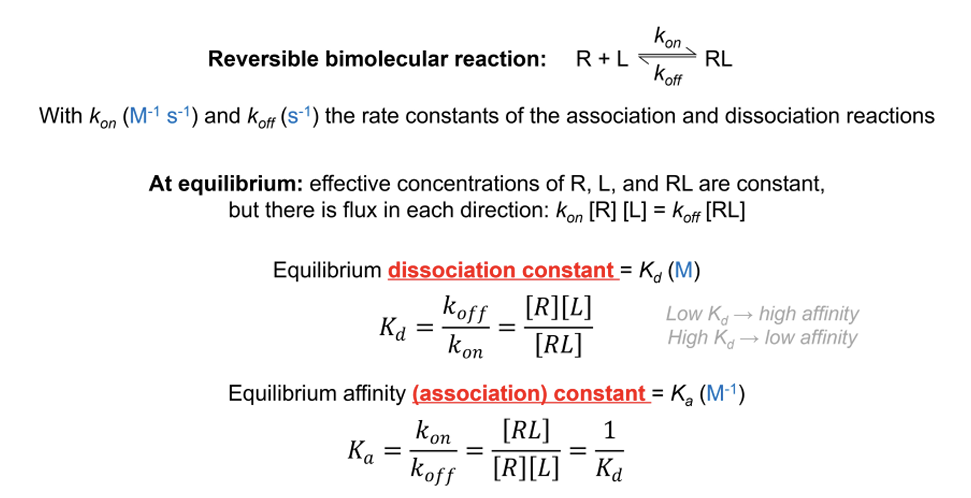
equilibrium dissociation constant (Kd),
the reverse of the equilibrium association constant (Ka)
equation for Kd and Ka
The smaller the Kd value …
the higher the binding affinity is, the faster ligand will binds and longer will stay in that conformation
the larger the Kd value ….
the smaller the binding affinity the longer it will take to bind and the fats the interaction will be complex will not last for long
why is knowing drug affinity important
important in drug design the larger the drug affinity is the longer it will bind to target and so only need a small dose and so limiting amount of side effects
most common way to determine the Kd value for a given receptor-ligand complex
vary concentration of Ligand in a fixed lowconcentration of receptor
At each concentration of ligand ([L]), the fraction of bound receptor or occupied binding sites (Θ) – is measured
Θ ranges form
0-1
when do we get Kd
when Θ is ½ then, [L] = Kd
So Kd is
Kd can therefore be interpreted as the ligand concentration that leads to 50% occupancy of the receptor’s binding site
Typical Kd values range from
10-15 M to 10-3 M
Chaperones
interact with non- native proteins (unfolded / partially folded or improperly folded proteins)
Assist protein folding by preventing non specific aggregation between protein
or provide microenvironemnt proteins
misfolding
have hydriphobic amino asids on the cell surface which can bind with other molecules causing aggregates
protein aggregation can lead to diseases like; sickle cell anemia, alshizmers, parkinsons , huntintons
Denaturing conditions include:
Temp
pH
chaotropic agents guanidinium ion and urea
Detergents
reducing agents
mechanical stress
Denaturing curve shows
relatively sharp transition from the folded, or native, form to the unfolded, or denatured, form in a sigmoid curve
half way in the curve
find temperature Tm where 50% folded and 50% unfolded
Kd= 1
from here we can determine ..
denaturant concentration at which the protein is 50% unfolded (C1/2).
We determine the temperature at which the protein is 50% unfolded (Tm or melting temperature)
Keq = 1
ΔG0 = 0