Chapter 12: Alkanes
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
What are alkanes?
Alkanes are saturated hydrocarbons — they contain only single C–C and C–H bonds.
General formula: CₙH₂ₙ₊₂
Derived from natural gas and crude oil; among the least reactive organic compounds.
Commonly used as fuels and lubricants, reacting with oxygen to release energy.
🔹 Bonding in Alkanes
Each carbon atom forms four sigma (σ) bonds via overlap of orbitals.
σ-bond = a shared pair of electrons with direct orbital overlap between two atoms.
Each C–C and C–H bond is a σ-bond → gives free rotation around each single bond.

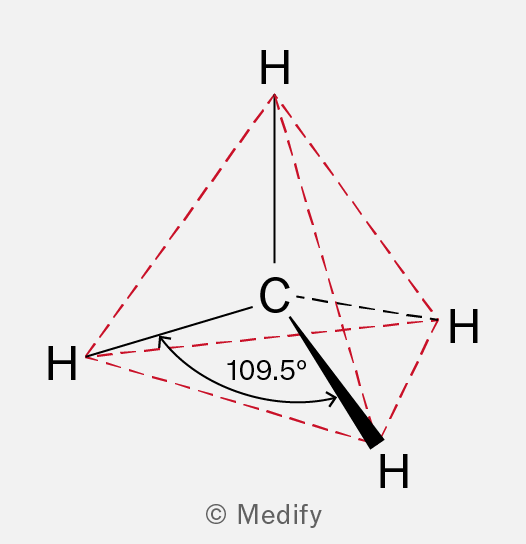
🔹 Shape of Alkanes
Each carbon in an alkane forms four σ-bonds arranged tetrahedrally (bond angle ≈ 109.5°), according to electron pair repulsion theory, to minimise repulsion between the electron pairs.
Methane (CH₄) is perfectly tetrahedral.
Larger alkanes have a zig-zag structure due to rotation around C–C bonds.
How do the general formula or branched or cyclic alkanes differ?
Alkanes can be branched but will still conform to the same general formula.
Cyclic alkanes, despite similarities in reactivity and properties to alkanes, form their own homologous series, with the same general formula as alkenes, .
Reactivity of Alkanes
Alkanes are the least reactive of all organic compounds.
Alkanes are composed of strong covalent bonds, which require a large amount of energy to break.
The similar electronegativity values of hydrogen and carbon means there is very low polarity in the carbon-hydrogen bonds. There are no electron-rich areas to attract electrophiles nor electron-deficient areas to attract nucleophiles.
Trend 1: Increasing Chain Length
As chain length increases, boiling point increases.
Reason: larger molecules = greater surface area → stronger London dispersion forces.
More energy is needed to overcome these forces.
Trend 2: Effect of Branching
Branched-chain isomers have lower boiling points than straight chains.
Branching → smaller surface area → fewer contact points → weaker London forces.
Cannot pack as closely together
Solubility & Density
The lack of molecular polarity in alkanes makes them insoluble in polar solvents, such as water.
The density of alkanes increases with chain length, and is always lower than water. When combined with an aqueous solution, the alkane will always form the upper layer.

Linear Alkanes
Linear alkanes have higher boiling points than their branched isomers. There is greater surface contact between linear molecules, resulting in closer packing and stronger London forces that require more energy to break.
Complete Combustion of Alkanes
CₓHᵧ + (x + y/4)O₂ → xCO₂ + (y/2)H₂O'
Alkanes burn readily in oxygen, producing carbon dioxide and water.
Making bonds releases energy to the environment; it is exothermic.
The high bond enthalpy of the carbon-oxygen bond, in the carbon dioxide product, results in a highly exothermic combustion reaction.
Alkanes are commonly used as fuels.
Incomplete combustion
When there is insufficient oxygen available, the carbon from the alkane is not fully oxidised.
This is incomplete combustion.
Incomplete combustion also results in the formation of carbon monoxide and/or particulate carbon (soot) alongside carbon dioxide and water.
The ratio or carbon based products will depend on the amount of oxygen available.
Incomplete combustion (carbon monoxide formed):
CₓHᵧ + (x/2)O₂ → xCO + (y/2)H₂O
Incomplete combustion (carbon/soot formed):
CₓHᵧ + (x/4)O₂ → xC + (y/2)H₂O
CO hazards
Hazards:
CO is toxic and colourless, binds irreversibly with haemoglobin forming carboxyhaemoglobin, reducing oxygen transport.
Alkane combustion pollutants
Alongside complete combustion products, other pollutants can be found within the exhaust fumes.
Additional pollutants including carbon monoxide, oxides of nitrogen and sulfur, carbon particulates, and unburned hydrocarbons like CH4 contributes to 22x CO2 global warming

All carbon based products of fuel combustion have negative environmental impacts.
Carbon dioxide emissions are linked to the greenhouse effect and global warming, and are present in both complete and incomplete combustion.
Carbon monoxide and particulate carbon are formed in incomplete combustion.
Carbon monoxide is a colourless, odourless, and toxic gas. It binds to haemoglobin in the blood more strongly than oxygen, reducing the capacity of the blood to transport oxygen around the body. Carbon monoxide poisoning is first noticeable through dizziness and can eventually lead to death.
Particulate carbon is linked to global dimming. It can settle on the leaves of plants, reducing photosynthesis, and can be inhaled, causing irritation to the lungs.
Sulfur dioxide causes air pollution and is linked to the formation of acid rain. It is very soluble and forms an acidic solution in rainwater.
🔹Free Radical Substitution
Alkanes undergo free radical substitution reactions with chlorine or bromine in the presence of UV radiation.
The radical substitution of an alkane with a halogen involves the homolytic fission of bonds and proceeds via three types of reaction;
Initiation – the formation of radicals from a non radical, in this case the halogen
UV light causes homolytic fission of Br₂ → 2 Br· radicals
Propagation – formation of a radical and non radical from a radical and non radical
1. CH₄ + Br· → ·CH₃ + HBr
2. ·CH₃ + Br₂ → CH₃Br + Br·
→ Chain reaction continues as Br· regenerated.
Termination – combination of two radicals to form a non radical product.
Two radicals combine → molecule forms, ending the chain.
Br· + Br· → Br₂
·CH₃ + ·CH₃ → C₂H₆
·CH₃ + Br· → CH₃Br
🔹 Further Substitution
Multiple H atoms may be replaced → CH₂Br₂, CHBr₃, CBr₄.
Radical substitution has limited use in organic synthesis as a mixture of products are formed.

Haloalkanes formed during free radical substitution are susceptible to repeated radical attack when the halogen is in excess. This leads to multiple substitutions .
Substitution at Different Positions
In longer alkanes, substitution can occur at different carbon atoms, producing structural isomers.
Example: C₅H₁₂ forms three monobromo isomers.
how to write mechanisms for free radical substitution of alkanes?

Methane reacts with chlorine in the presence of UV light to form a mixture of chlorinated alkanes.
The products formed depend on the concentration of the reactants present.
If excess chlorine is used with methane as the limiting reagent, then further substitution reactions can occur, forming di, tri, and tetrachloro products.
An excess of methane and limiting reagent of chlorine ensures that the monosubstituted chloromethane is the major product.
⚠ Limitations of Radical Substitution
Produces mixtures of products → lack of selectivity.
Difficult to control → limits synthetic usefulness.