120 - Revised Joints and Muscles
1/118
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
119 Terms
Joints (2)
Aka articulation, arthrosis
Attach bone to bone (ligaments), bone x cartilage, bone x teeth connection
Fibrous
Cartilaginous
Synovial
Types of Joints (based on material/manner of connection) [3]
Fibrous (3)
[TYPE OF JOINT]
Dense irregular CT; immovable to slightly movable
Sutures
Syndesmoses
Cartilaginous
[TYPE OF JOINT]
Solid hyaline/fibrous cartilage; little to no movement
Synchondrosis
Symphyses
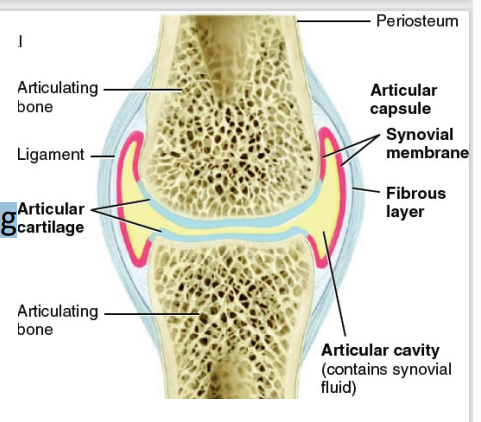
Synovial (2)
[TYPE OF JOINT]
Two-layered articular capsule uniting articulating bones & surrounds lubricated space (articular cavity)
Movement: slight to free
![<p><span style="color: red"><strong>[TYPE OF JOINT]</strong></span></p><ul><li><p>Two-layered articular capsule uniting articulating bones & surrounds lubricated space (articular cavity)</p></li><li><p>Movement: slight to free</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/60ea3477-c846-4f44-934a-7821e240a0ed.png)
A. Sutures
Skull
A. Sutures
B. Syndesmoses
C. Synchondrosis
D. Symphyses
false - it does not
T/F - the skull offers movements
B. Syndesmoses
greater distance between articulating components & more dense irregular CT
A. Sutures
B. Syndesmoses
C. Synchondrosis
D. Symphyses
Interosseus ligament
Interosseus membrane
Gomphosis (connected by periodontal ligament)
Syndesmoses [3]
C. Synchondrosis
connected by solid piece of hyaline/fibrous cartilage
A. Sutures
B. Syndesmoses
C. Synchondrosis
D. Symphyses
Rib-sternum
Epiphyseal cartilage (growth centers) → synostosis
Synchondrosis [2]
D. Symphyses
bone ends covered in hyaline; connected by flat disk of fibrous
A. Sutures
B. Syndesmoses
C. Synchondrosis
D. Symphyses
Pubic symphyses
Sternum
Intervertebral joints
Symphyses [3]
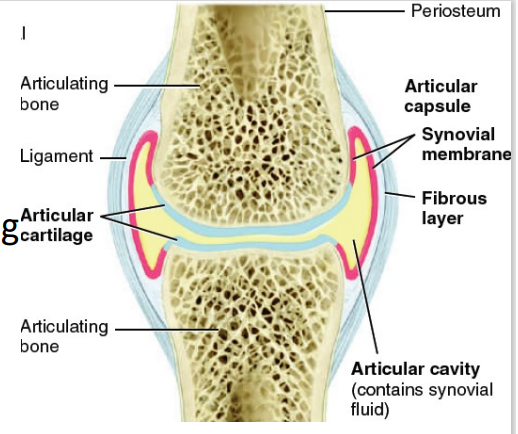
Articular cartilage
Articular cavity
Articular Capsule
Outer fibrous layer
Inner synovial membrane
Synovial fluid
Accessory ligaments, articular discs, labra
Nerve and blood supply
Bursae, tendon sheath
Parts of Synovial Joint [7]
![<p>Parts of Synovial Joint<span style="color: red"> <strong>[7]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/227ef0f2-cc97-4227-bca4-ce4e3fa04bbc.png)
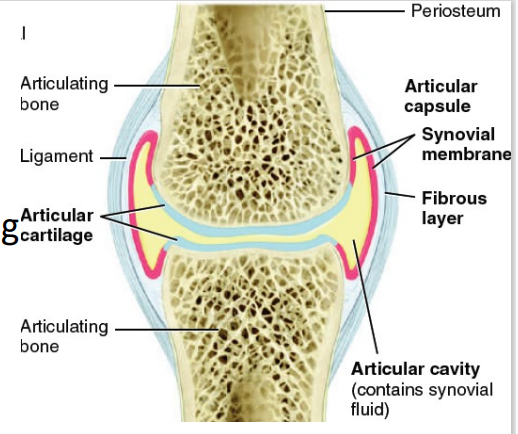
Articular cartilage
[PART OF SYNOVIAL JOINT]
layer of hyaline cartilage covering bone (friction, shock absorption)
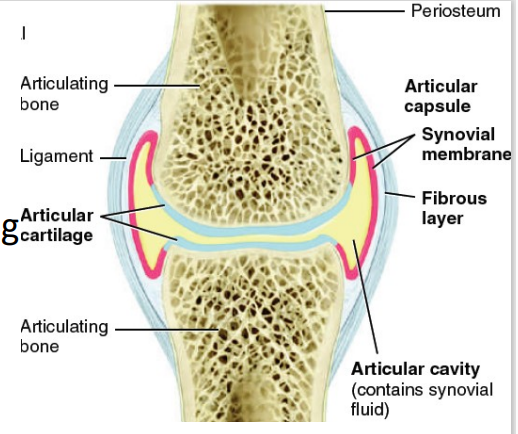
Articular cavity
[PART OF SYNOVIAL JOINT]
space between bones
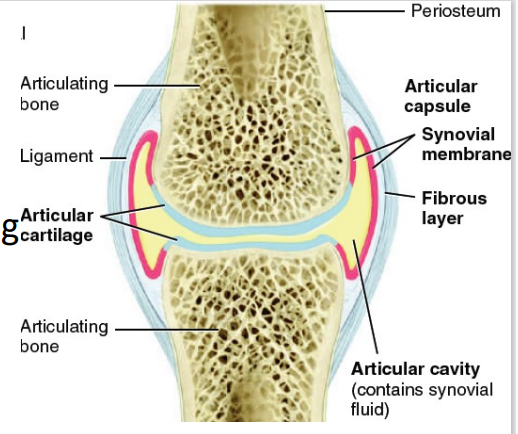
Articular capsule (3)
[PART OF SYNOVIAL JOINT]
surrounds joint and encloses cavity
Outer fibrous layer (periosteum extension)
Inner synovial membrane
D. Both statements are false.
I. Outer fibrous layer (periosteum extension) – allows for freer movement
II. Inner synovial membrane – areolar CT + collagen + elastic fibers + articular fat pads
[PART OF SYNOVIAL JOINT]
I. Inner synovial membrane – allows for freer movement
II. Outer fibrous layer (periosteum extension) – areolar CT + collagen + elastic fibers + articular fat pads
A. The first statement is true. The second statement is false.
B. The first statement is false. The second statement is true.
C. Both statements are true.
D. Both statements are false.
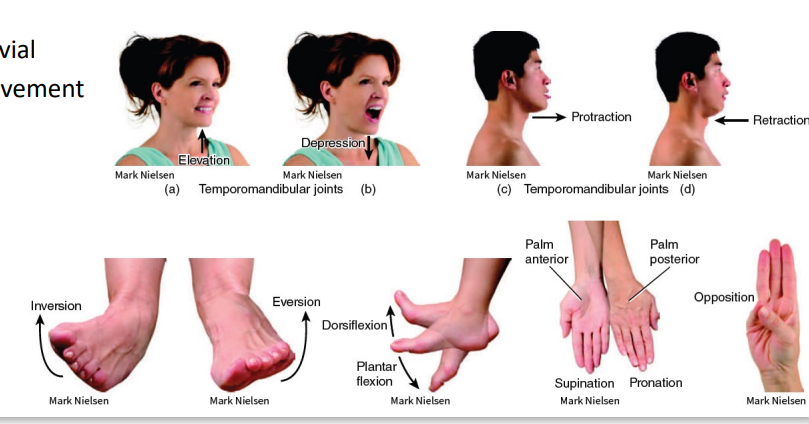
Synovial (Movement) - Flexion and Extension
*just read/study
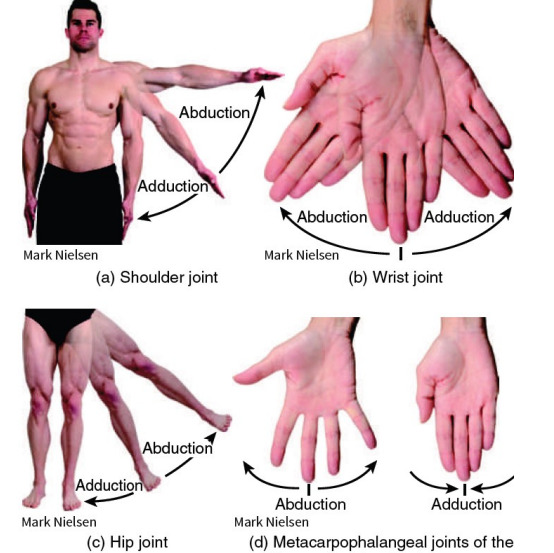
Abduction - away from you (moving away from the midline)
Adduction - inward (toward the midline)
Synovial (Movement) - Abduction and Adduction
*just read/study
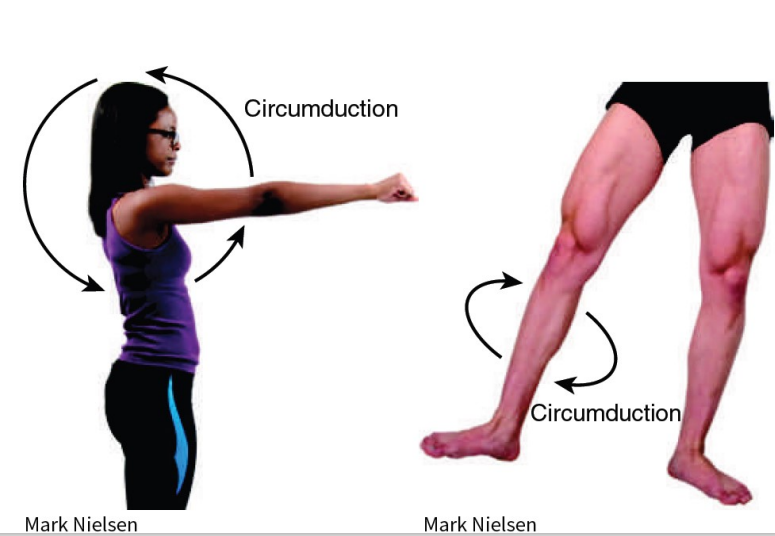
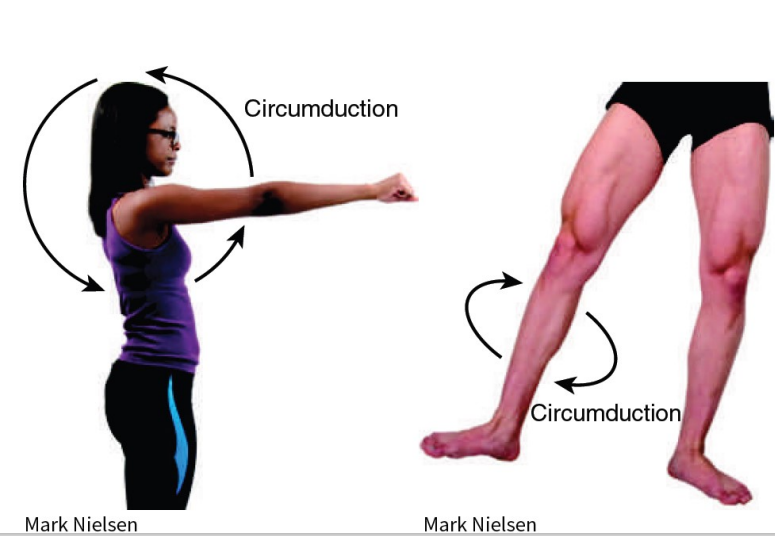
Synovial (Movement) - Circumduction
*just read/study

Synovial (Movement) - Lateral and Medial Rotation
*just read/study

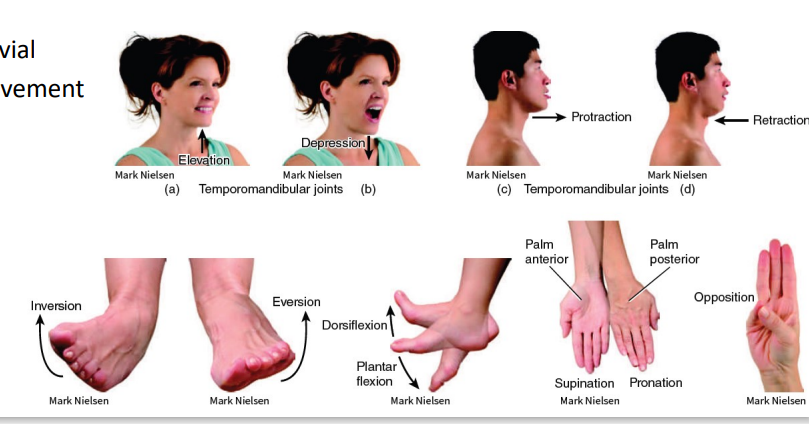
Synovial (Movement)
*just read/study
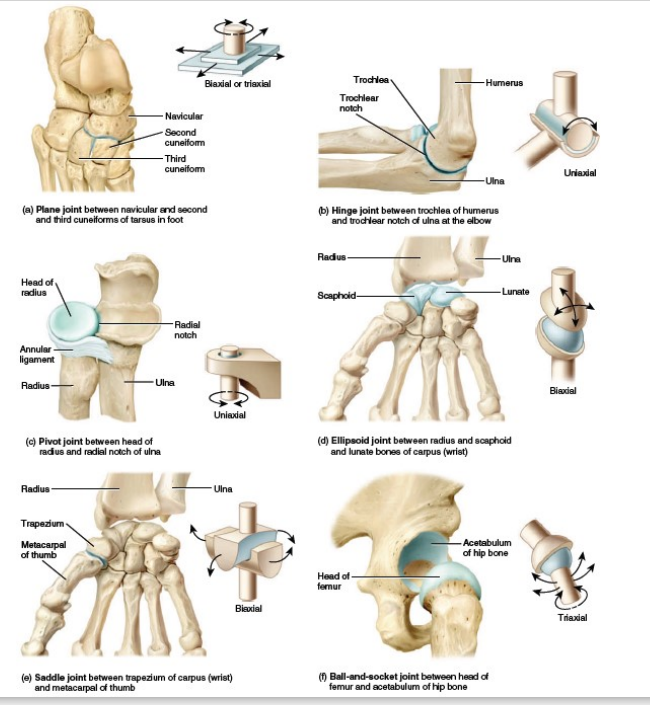
Plane
Hinge
Pivot
Ellipsoid
Ball-and-socket
Types of Synovial Joints [5]
*DAANAN LANG DAW SABI NI SIR I GUESS NOT SO IMPORTANT
Oestoarthritis
Rheumatoid Arthritis
Gouty Arthritis
Types of Arthritis Joint Conditions (3)
B. The first statement is false. The second statement is true.
Osteoarthritis - cartilage destruction
before it was wear and tear but problem lies in the incompetence of the tissue in replacing its damaged components.
I. Osteoarthritis - wear and tear of the cartilage
II. Rheumatoid arthritis - autoimmune
A. The first statement is true. The second statement is false.
B. The first statement is false. The second statement is true.
C. Both statements are true.
D. Both statements are false.
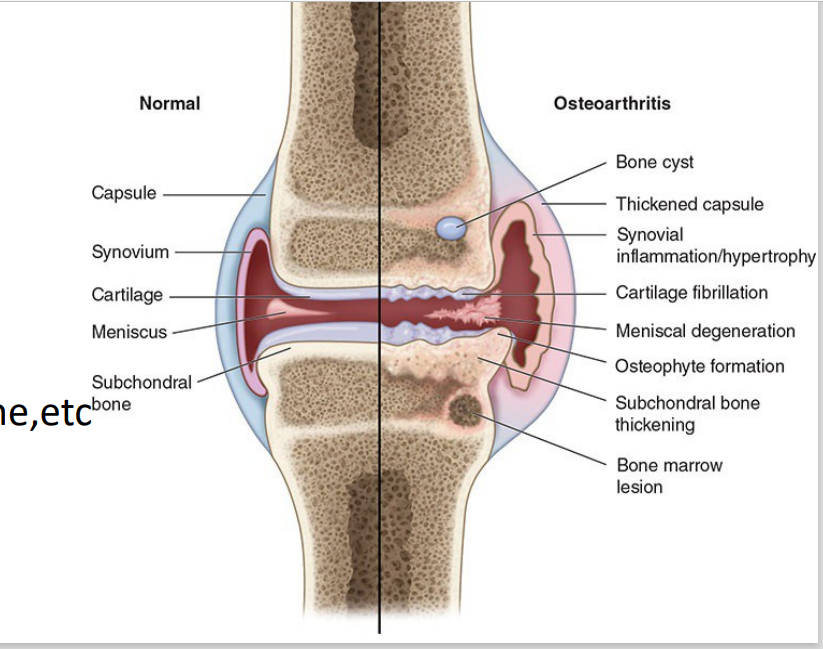
Cartilage provides lubrication, shock absorption, compressibility, and friction-free movement
Cartilage turnover regulated by GFs (BMP-2, IGF-1, matrix metalloproteinases)
Chrondrocytes removed damaged cartilage, increase synthesis of ECM
Supported by tendons, ligaments, subchondral bone,etc
Nourished by synovial fluid
Normal conditions (w/o Osteoarthritis) [5]
Chondrocytes
Removes damaged cartilage, increase synthesis of ECM
Cartilage
Provides lubrication, shock absorption, compressibility, and friction-free movement in the joints.
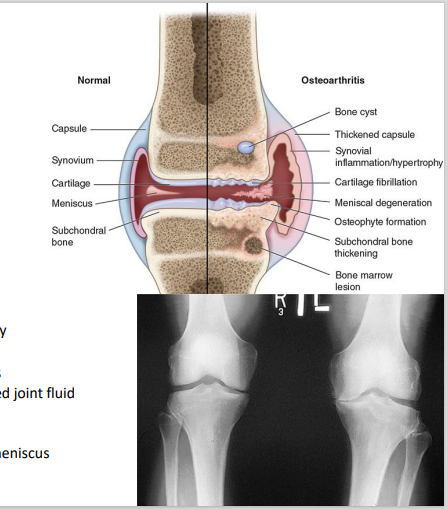
Osteoarthritic cartilage
Articular damage (trauma, overload, etc)
Chondrocytes eventually overwhelmed (breakdown >>> resynthesis)
Cartilage slowly eroded, exposing subchondral bone
Brittle, stiffer, less weight-bearing capacity
Inflammation
bone will grind to other bone coz cartilage eroded → become bone fragments → inflammation
Pain – nerve endings activated by irritants
Distension of synovial capsule by increased joint fluid
Microfractures
Periosteal irritation
Damage to ligaments, synovium, or the meniscus
Condition of joints (w/ Osteoarthritis) [5]
No Medication for Chondrocytes or address its inability to resynthesize, only pain management
Medications to support chondrocytes
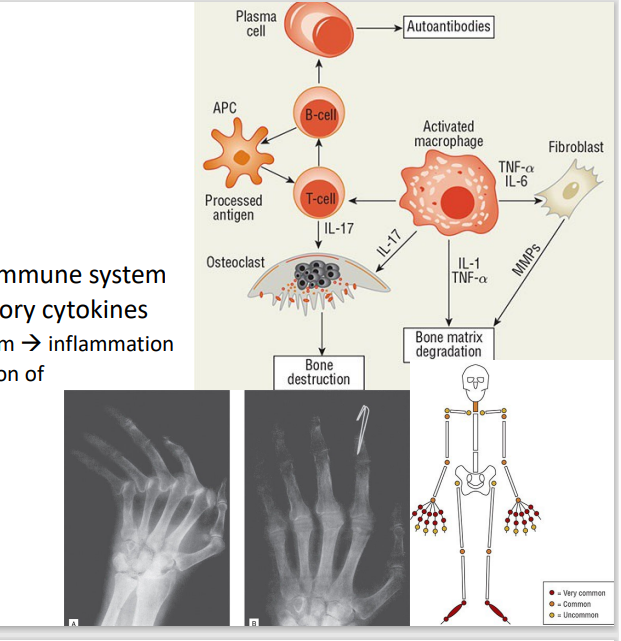
Rheumatoid Arhtritis
[Joint Conditions: Arthtritis]
Overstimulation of the innate immune system
Autoantibodies, pro-inflammatory cytokines
Immune cells migrate to synovium → inflammation
IL-6 & TNF-α – promote expression of matrix metalloproteinases
Usually bilateral
C. Both statements are true.
I. IL-6 & TNF-α – promote expression of matrix metalloproteinases
II. Too much of this can lead to rheumatoid arthritis.
A. The first statement is true. The second statement is false.
B. The first statement is false. The second statement is true.
C. Both statements are true.
D. Both statements are false.
Concentration & solubility
Temperature
Nucleation and growth
Note:
Concentration and Solubility: Uric acid is less soluble at higher concentrations and lower pH. More Uric Acid → precipitation
Temperature: Crystals are more likely to form in cooler parts of the body (e.g., distal joints like toes).
Nucleation and Growth: Uric acid forms MSU crystals, which grow and trigger an immune response. excessive trauma = ↑ risk for crystallization
Factors that affect crystal formation in Gouty Arthritis [3]
Uric Acid
purine metabolism byproduct
purine metabolism byproduct, forms crystals when its concentration exceeds solubility limits
cooler areas like the metatarsophalangeal joint (big toe, podagra)
Area which uric acid crystals tend to form more
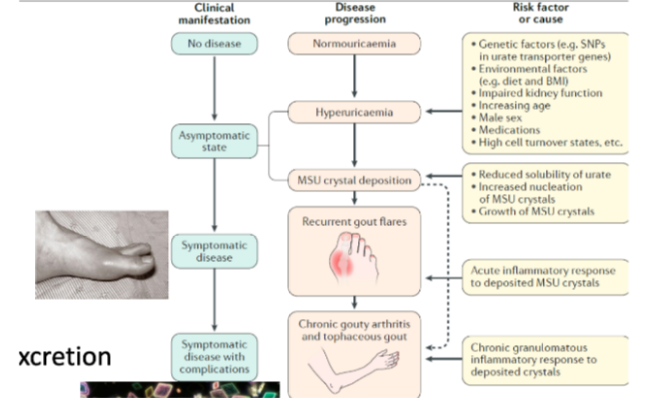
Gouty Arthritis
[Joint Conditions: Arthritis]
is an inflammatory condition caused by the deposition of monosodium urate (MSU) crystals in joints coz of hyperuricemia from uric acid overproduction or underexcretion.
Monosodium urate + inflammasome + immune cell recruitment
Gouty Arthritis is a combination of?
Crystals attract and promote the assembly of inflammasomes (cytosolic multiprotein complexes).
Inflammasomes activate enzymes that lead to the activation of IL-1β.
Immune cells attack the crystals as part of the response.
What is the role of crystals in inflammasome activation and immune response?
IL-1β
Vasodilation
Recruitment of monocytes, neutrophils
Production of matrix-degrading enzymes
[FILL IN THE BLANK]
In Gouty Arthritis, crystal deposition triggers inflammation via the NLRP3 inflammasome, releasing cytokines like [BLANK] and recruiting neutrophils, resulting in intense pain, redness, and swelling.
No, unsalted vegetables with purines (e.g., mung beans), nuts, beans, and tofu are generally safe for gout patients. Instead, advise reducing salty foods (high sodium can precipitate monosodium urate) and being cautious with meat purines. Trigger foods like alcohol and sugary drinks should also be avoided. A strict no-purine diet is not recommended as it is unpalatable.
Should a patient with gout avoid mung beans, tofu, or other vegetables high in purines?
Podagra
[FILL IN THE BLANK]
Gout typically presents as acute monoarticular arthritis, affecting a single joint. The most common joint is the metatarsophalangeal (MTP) joint of the big toe, referred to as [BLANK].
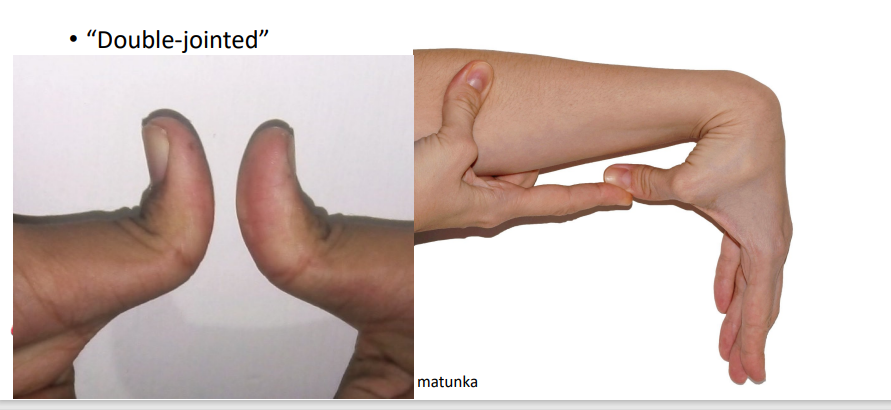
it is not two joints, just anatomy that allows it
Different from Ehlers-Danlos Syndrome where the joint connectivities are already loose w/ symptoms of loose joints, skin and connective tissue
Double jointed
popping of bubbles in synovial fluid
does not damage the joint
Cracking Knuckles
Tendinopathy (Tendonitis)
[Joint Conditions]
A tendon injury (tendon tear/ rupture) that may cause chronic or acute pain and is not associated with inflammation other than tendon tear/ rupture
Potential causes include metallics and metalloproteinases.
What are potential causes of tendon injury (theory)?
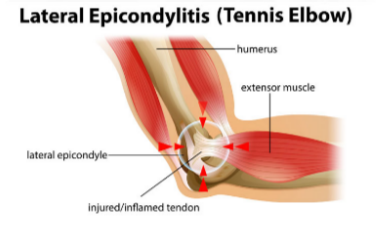
Elbow tendinopathy - no inflammation
(Tx: reduce load on elbow, less stretching, ensuring pads cover the elbow)
[Joint Conditions]
(formerly elbow epicondylitis)
Degeneration from repeated mechanical overload (e.g. heavy lifting, tennis)
Epicondyles
bony prominences on medial and lateral sides of distal humerus (upper arm bone
does not target because there is no inflammation
Why are NSAIDs not recommended for Tennis Elbow?
Fluoroquinolone Tendinopathy
Damage is rare but tends to be permanent
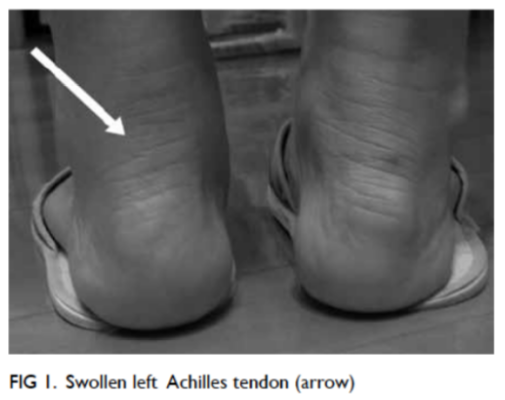
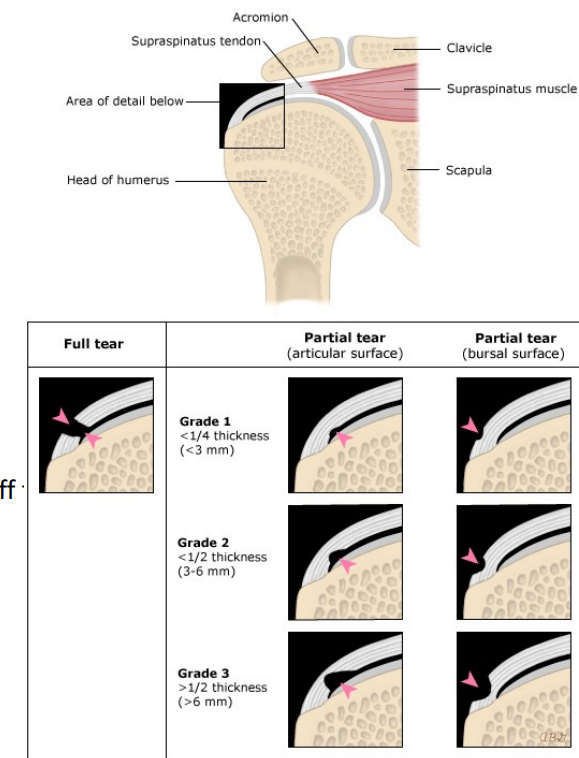
Frozen shoulder/adhesive capsulitis
[Joint Conditions]
Inflammation, adhesion/fibrosis of synovial lining (scarring)
Risk factors: T2DM, shoulder injuries (ex. rotator cuff injury)
Rotator cuff
When ability of [BLANK] to maintain joint stability and compress overwhelmed by external forces (throwing activity, trauma, overuse)
C. Both statements are true.
Common Manifestations: Sprain = tapilok ; Strain = stiff neck
I. Sprain – forcible wrenching/twisting of ligaments without bone dislocation due to overtwisting
II. Strain – stretched/partially torn tendon/ muscle due to improper position
A. The first statement is true. The second statement is false.
B. The first statement is false. The second statement is true.
C. Both statements are true.
D. Both statements are false.
Movement
Positioning
Storage and movement (sphincters, smooth muscle)
Thermogenesis
Functions of Muscular Tissue (Skeletal)? [4]
Electrical excitability – can respond to stimuli by producing action potential
Autorhythmic electrical signals (cardiac pacemaker)
Chemical stimuli (NTs, hormones, local pH change)
Contractility (forceful) – when stimulated
Contraction generates tension (force of contraction)
Extensibility – stretch without damage, to an extent
Elasticity – return to original shape
Properties of Muscular Tissue [4]
A. The first statement is true. The second statement is false.
SQ/hypodermis (areolar CT) ; Fascia (dense irregular CT)
I. Each muscle is an organ composed of muscle fibers
II. SQ/hypodermis = dense irregular CT ; Fascia = areolar CT
A. The first statement is true. The second statement is false.
B. The first statement is false. The second statement is true.
C. Both statements are true.
D. Both statements are false.
Subcutaneous (SQ) Tissue/Hypodermis (areolar CT)
Fascia (dense irregular CT)
Connective Tissue (CT) Components in Muscles [2]
Subcutaneous (SQ) Tissue/Hypodermis
[Connective Tissue (CT) Components in Muscles]
Separates muscle from skin
Pathway for nerves, blood vessels, and lymphatic vessels to enter/exit muscle
Triglyceride storage
Fascia
[Connective Tissue (CT) Components in Muscles]
Groups muscles with similar functions
Also carries nerves, blood vessels, lymphatics
Layers: epimysium (outer, encircling), perimysium (bundles fibers into fascicles/10-100 fibers), endomysium (separates individual fibers/cells)
epimysium (outer, encircling)
perimysium (bundles fibers into fascicles/10-100 fibers)
endomysium (separates individual fibers/cells)
Layers in Fascia [3]
Sarcolemma
T tubules
Sarcoplasm
Structure of Muscle cell/Muscle fiber [3]
A. Sarcolemma
[Structure of Muscle cell/Muscle fiber]
fiber plasma membrane
A. Sarcolemma
B. T tubules
C. Sarcoplasm
B. T tubules
[Structure of Muscle cell/Muscle fiber]
invaginations on sarcolemma (action potential travels through)
A. Sarcolemma
B. T tubules
C. Sarcoplasm
C. Sarcoplasm
[Structure of Muscle cell/Muscle fiber]
fiber cytoplasm
Lots of glycogen
Myoglobin, Sarcoplasmic reticulum, Myofibrils
A. Sarcolemma
B. T tubules
C. Sarcoplasm
A. Myoglobin
[Sarcoplasm]
binds O2 for mitochondrial use
A. Myoglobin
B. Sarcoplasmic reticulum
C. Myofibrils
B. Sarcoplasmic reticulum
[Sarcoplasm]
(~smooth ER) – stores Ca2+ (relaxed muscles)
A. Myoglobin
B. Sarcoplasmic reticulum
C. Myofibrils
C. Myofibrils
[Sarcoplasm]
contractile organelles
Myofilaments (small protein structures arranged in sarcomeres) – thin filaments (made of actin) + thick filaments (made of myosin)
A. Myoglobin
B. Sarcoplasmic reticulum
C. Myofibrils
D. Both statements are false.
thin filaments (made of actin) + thick filaments (made of myosin)
I. In myofilaments, thin filaments are made up of myosin
II. While thick filaments are made up of actin
A. The first statement is true. The second statement is false.
B. The first statement is false. The second statement is true.
C. Both statements are true.
D. Both statements are false.
C. Both statements are true.
I. Myofibrils are arranged in sarcomere compartments)
II. Sarcomeres separated by Z discs (dense protein)
A. The first statement is true. The second statement is false.
B. The first statement is false. The second statement is true.
C. Both statements are true.
D. Both statements are false.
Actin
Myosin
Regulatory proteins (thin filament)
Structural proteins
Proteins in Myofibrils [4]
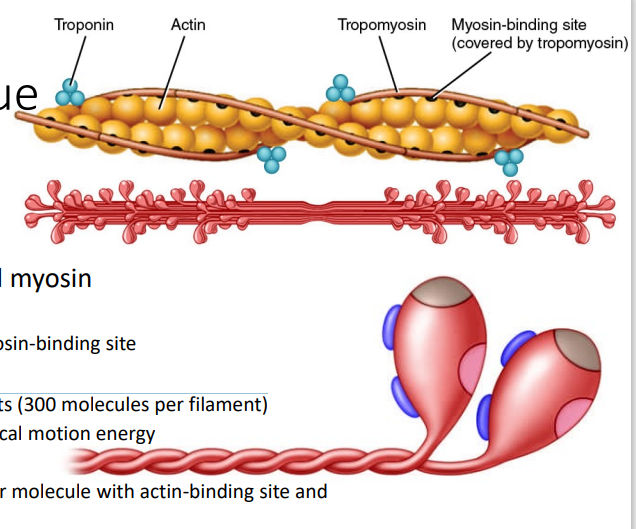
Actin
[MYOFIBRILS - PROTEINS]
Thin filament proteins with myosin-binding site
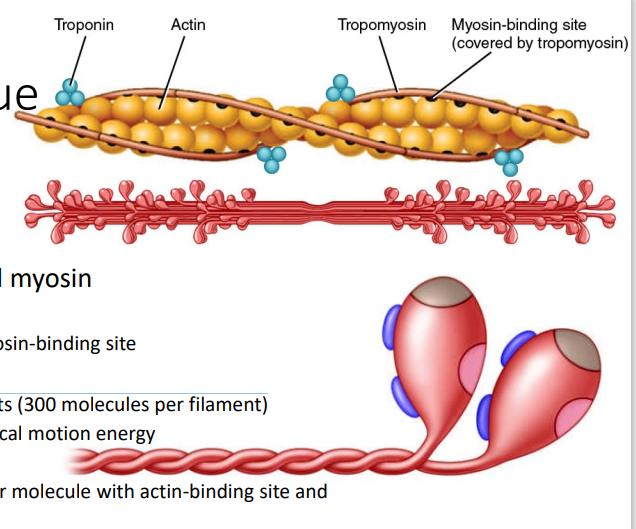
Myosin
[MYOFIBRILS - PROTEINS]
Motor proteins in thick filaments (300 molecules per filament)
Convert ATP energy to mechanical motion energy
Myosin tail
Myosin head – 2 projections per molecule with actin-binding site and ATP-binding site/ATPase
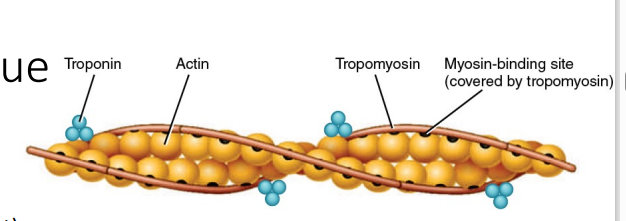
Tropomyosin – covers myosin-binding site when muscles relaxed •
Troponin – complex of 3 proteins (Costanzo)
Troponin T (“T” = tropomyosin) attaches the troponin complex to tropomyosin.
Troponin I (“I” for inhibition) inhibits interaction of actin and myosin.
Troponin C (“C” = Ca2+) binds Ca2+ during muscle contraction; causes conformational change to displace tropomyosin
[MYOFIBRILS - PROTEINS]
What are the two regulatory proteins?
Titin
α-actinin
Myomesin
Nebulin
Dystrophin
[MYOFIBRILS - PROTEINS]
What are the 5 structural proteins in myofibrils?
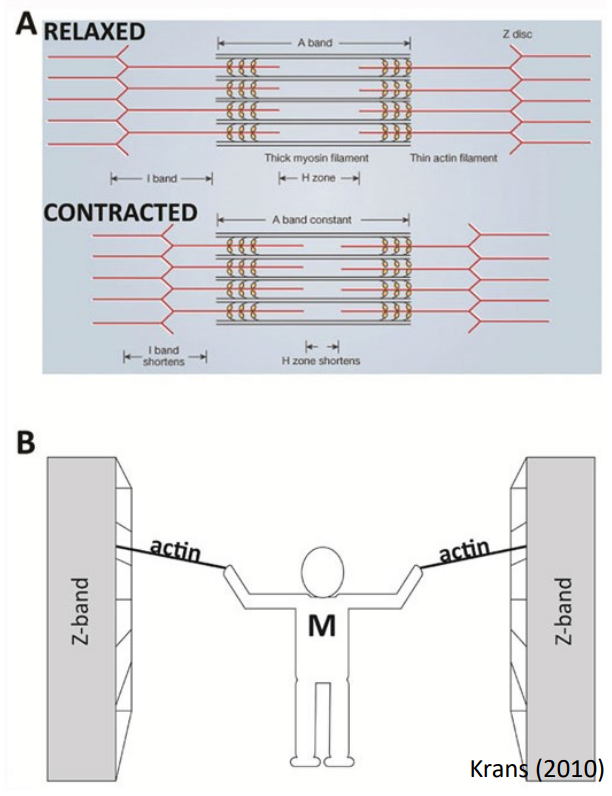
Myosin head (thick filament) “walks” on actin in thin filaments
Starts with an action potential
Contraction – Sliding Filament Theory [2]
Neuromuscular Junction (NMJ)
Somatic motor neuron
End: axon terminals dividing into somatic end bulbs
Synaptic cleft
Muscle fiber (Motor end plate
[CONTRACTION: Action Potential Generation]
is the site where a somatic motor neuron communicates with a skeletal muscle fiber (motor end plate) to initiate contraction.
Synaptic cleft
[NEUROMUSCULAR JUNCTION]
A small gap between the synaptic end bulb and the muscle fiber's membrane (sarcolemma).
Neurotransmitters (e.g., ACh) are released into this cleft to bridge the gap and transmit the signal.
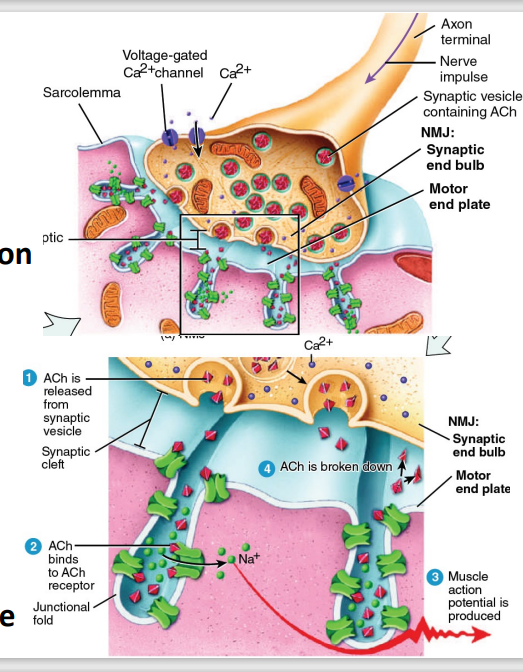
Generated at the Neuromuscular Junction (NMJ)
Acetylcholine (ACh) stored in vesicles
Action potential → Ca2+ influx
→ Vesicle exocytosis → ACh release
ACh binds to motor end plate receptors (Nm: ligand-gated ion channels)
Na+ , cation inflow → inside more +++
ACh metabolized by acetylcholinesterase
Release of ACh:
A nerve impulse triggers the opening of voltage-gated calcium (Ca²⁺) channels in the synaptic end bulb.
Calcium ions enter the axon terminal, prompting synaptic vesicles to release ACh into the synaptic cleft via exocytosis.
ACh Binding to Receptors:
ACh diffuses across the synaptic cleft and binds to ACh receptors on the motor end plate.
These receptors are Nm ligand-gated ion channels that open upon ACh binding.
Sodium Ion (Na⁺) Influx:
The opened ion channels allow Na⁺ to flow into the muscle fiber, leading to depolarization of the sarcolemma at the motor end plate.
This depolarization generates an end-plate potential, which propagates as a muscle action potential.
Breakdown of ACh:
To prevent continuous stimulation, ACh is broken down by the enzyme acetylcholinesterase (AChE) into acetate and choline.
Choline is recycled back into the neuron for ACh synthesis.
Contraction: Action Potential Generation [7]
read na lang to ayos pa
![<p>Contraction: Action Potential Generation <strong>[7]</strong></p><p><strong><em>read na lang </em>to ayos pa</strong></p>](https://knowt-user-attachments.s3.amazonaws.com/4c7b2b84-7b9c-49d1-bfc2-68cacebe730c.png)
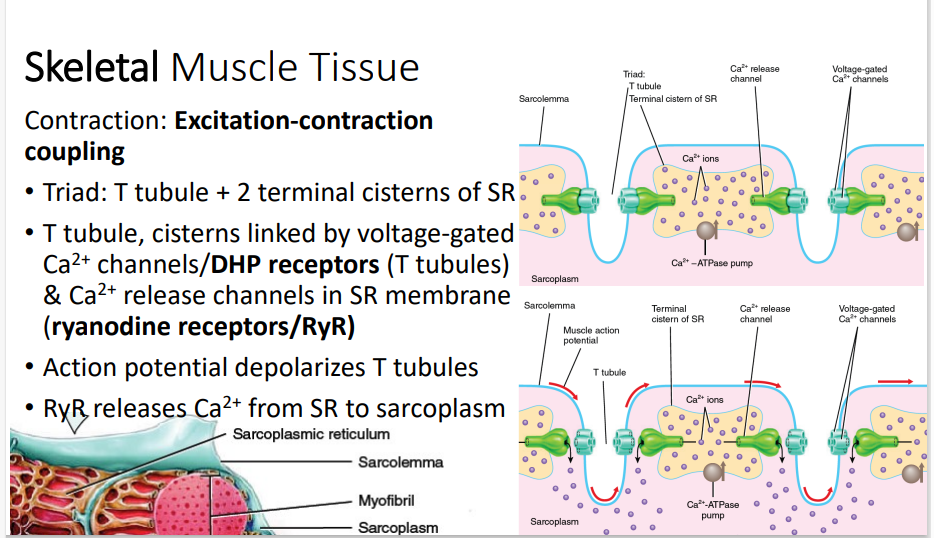
Triad: T tubule + 2 terminal cisterns of SR
T tubule, cisterns linked by voltage-gated Ca2+ channels/DHP receptors (T tubules) & Ca2+ release channels in SR membrane (ryanodine receptors/RyR)
Action potential depolarizes T tubules
RyR releases Ca2+ from SR to sarcoplasm
SUMMARY: Action Potential → Sarcolemma → Depolarization DHP receptors (T-tubules) → (+) RyR channels (SR) → Ca²⁺ released to sarcoplasm → bind to Troponin C → conformational change of Troponin → Tropomyosin moves → Exposes myosin-binding sites on actin → Myosin heads → Bind to actin → Initiates sliding filament mechanism = Muscle contraction → cross bridging cycle may begin
After Contraction → Ca²⁺ reuptake (via active transport by Ca²⁺-ATPase) → Ca²⁺ pumped back into SR = Reduced sarcoplasmic Ca²⁺ = Muscle relaxes
1. Triad Structure:
Triad: Composed of one T-tubule (transverse tubule) and two terminal cisternae of the sarcoplasmic reticulum (SR).
Purpose: The triad allows efficient communication between the T-tubules and the sarcoplasmic reticulum to trigger calcium release.
2. Action Potential Depolarizes T-Tubules:
An action potential generated at the sarcolemma propagates down the T-tubules.
This depolarization activates DHP receptors (voltage-sensitive dihydropyridine receptors) located on the T-tubules.
3. Coupling with the Sarcoplasmic Reticulum:
DHP Receptors are mechanically linked to RyR (ryanodine receptors), which are calcium-release channels in the SR membrane.
When DHP receptors sense depolarization, they trigger the opening of RyR channels.
4. Calcium Ion Release:
RyR Channels release stored Ca²⁺ ions from the sarcoplasmic reticulum into the sarcoplasm.
The increase in sarcoplasmic Ca²⁺ concentration is essential for initiating muscle contraction.
5. Calcium's Role in Contraction:
The released Ca²⁺ ions bind to troponin, which is part of the thin filament (actin).
This binding causes a conformational change that moves tropomyosin, exposing myosin-binding sites on actin.
The myosin heads then bind to actin, leading to the sliding filament mechanism and muscle contraction.
6. Calcium Reuptake:
After the contraction signal ends, Ca²⁺-ATPase pumps in the SR membrane actively pump Ca²⁺ back into the SR.
This reduces sarcoplasmic Ca²⁺ levels, allowing the muscle to relax.
Contraction: Excitation-contraction coupling [4]
calcium-channel blockers.
T-tubules are targeted by [BLANK]
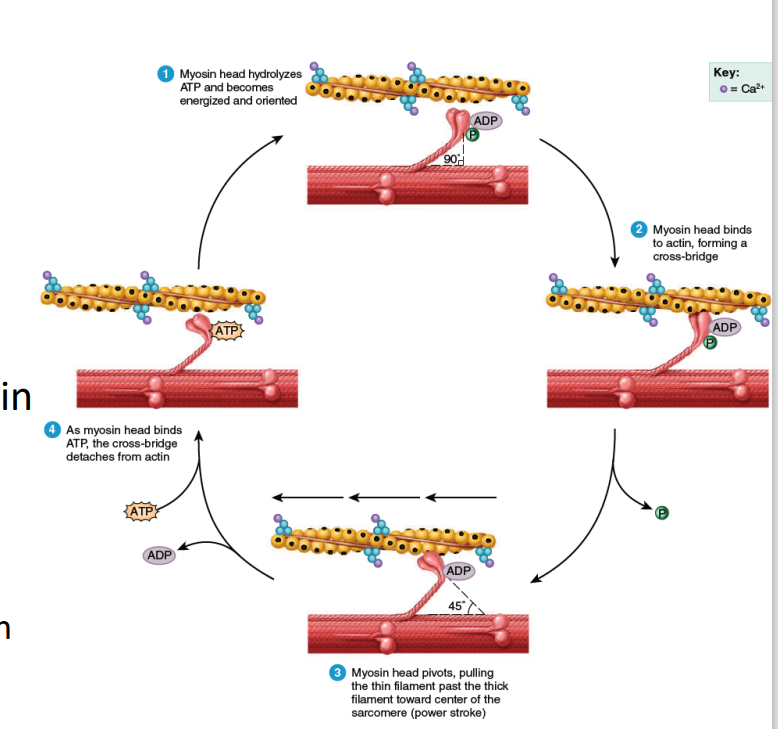
Myosin (with stored energy from ATP from previous contraction) binds to actin
Pi from previous contraction released
Bound myosin = cross-bridge
Power stroke
Myosin pivots (90o to 45o ), pulling thin actin filaments and generating tension
ADP from previous contraction released
ATP hydrolysis
ATP binds to myosin ATP-binding site; bound myosin head removed (What if no more ATP?)
Myosin’s ATP-binding site/ATPase hydrolyzes ATP (ADP + Pi) & stores energy
SUMMARY:
Step 1 - ATP Hydrolysis: ATP → hydrolysis via ATPase→ ADP + Pi → Myosin Head is energized → aligns head at 90° to the thick filament
Step 2 - Cross-Bridge Formation: Energized myosin head binds to actin → Cross-bridge formed (initial attachment b/t thick and thin filaments)
Step 3 - Power Stroke: Myosin head pivots from 90° → 45° → Pulls actin filament toward center of sarcomere → shorten muscle fiber → tension generated → ADP + Pi released
Step 4 - Detachment and Resetting: ATP binds to myosin head → Myosin head detaches from actin → Cycle repeats as long as Ca²⁺ is present
ATP Hydrolysis and Myosin Head Orientation (Step 1):
The myosin head, a part of the thick filament (shown in pink), hydrolyzes ATP into ADP and inorganic phosphate (Pi). This process energizes the myosin head and aligns it at a 90° angle to the thick filament, preparing it to bind to actin.
Cross-Bridge Formation (Step 2):
The energized myosin head binds to actin (the thin filament, shown in yellow), forming a cross-bridge. This is the initial attachment between the thick and thin filaments, crucial for muscle contraction.
Power Stroke (Step 3):
After the myosin head binds to actin, it undergoes a conformational change, causing it to pivot at a 45° angle. This pivoting action pulls the actin filament toward the center of the sarcomere, shortening the muscle fiber (this is the "power stroke"). The ADP and Pi are released during this motion.
Detachment and Resetting of Myosin Head (Step 4):
After the power stroke, ATP binds to the myosin head. This binding causes the myosin head to detach from actin, resetting for another cycle. The ATP is then hydrolyzed again, and the process can repeat as long as calcium ions (Ca²⁺) remain present to facilitate the interaction between actin and myosin.
Contraction – Cross-bridge cycling / contraction cycle
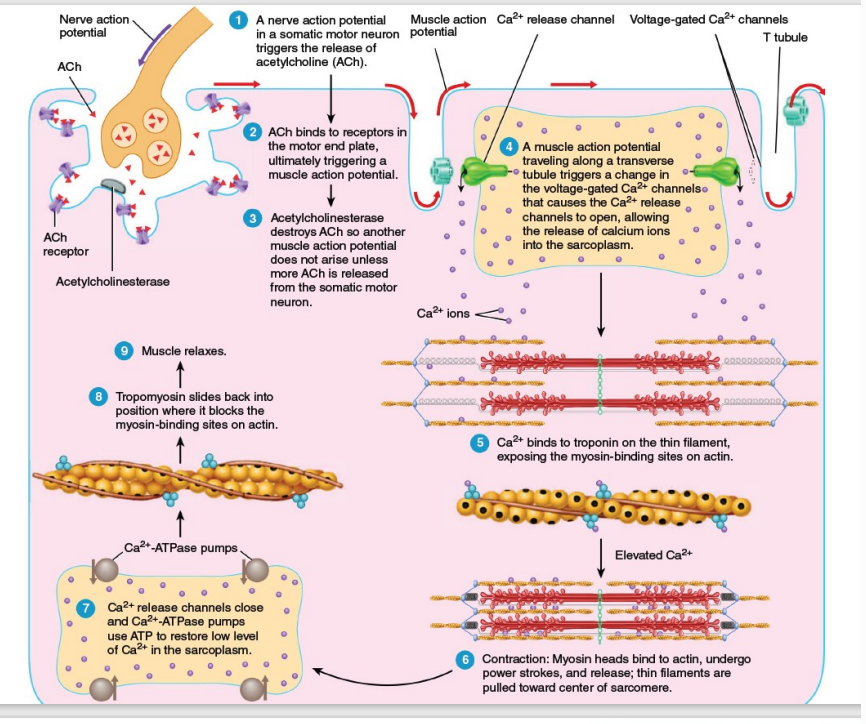
Nerve Action Potential and Acetylcholine (ACh) Release:
A nerve action potential arrives at the motor neuron terminal, causing the release of the neurotransmitter acetylcholine (ACh) into the synaptic cleft.
Binding of ACh to Receptors:
ACh diffuses across the synaptic cleft and binds to receptors on the motor end plate of the muscle fiber, initiating a muscle action potential.
ACh Breakdown:
Acetylcholinesterase, an enzyme in the synaptic cleft, breaks down ACh, ensuring the signal is brief and preventing continuous stimulation of the muscle.
Propagation of the Muscle Action Potential:
The muscle action potential travels along the sarcolemma (muscle cell membrane) and down into the T-tubules, triggering the opening of voltage-gated calcium (Ca²⁺) channels in the sarcoplasmic reticulum.
Calcium Release and Binding to Troponin:
Ca²⁺ ions are released from the sarcoplasmic reticulum into the cytosol. These ions bind to troponin, a regulatory protein on the thin filament, causing tropomyosin to move and expose myosin-binding sites on actin.
Crossbridge Formation and Contraction:
With the myosin-binding sites exposed, myosin heads attach to actin, forming crossbridges. ATP hydrolysis powers the myosin heads to perform power strokes, pulling the thin filaments toward the center of the sarcomere, resulting in muscle contraction.
Calcium Reuptake:
After contraction, Ca²⁺-ATPase pumps actively transport Ca²⁺ back into the sarcoplasmic reticulum, reducing cytosolic calcium levels.
Tropomyosin Blocks Myosin-Binding Sites:
As Ca²⁺ levels decrease, tropomyosin returns to its resting position, blocking the myosin-binding sites on actin.
Muscle Relaxation:
The muscle relaxes as crossbridge cycling stops and the sarcomere returns to its resting length.
Nerve Action Potential → Arrives at the motor neuron terminal.
Motor neuron terminal → Causes release of Acetylcholine (ACh) into the synaptic cleft.
ACh → Diffuses across the synaptic cleft.
ACh → Binds to receptors on the motor end plate of the muscle fiber.
Motor end plate → Initiates muscle action potential.
Acetylcholinesterase (in synaptic cleft) → Breaks down ACh.
ACh breakdown → Ensures brief signal duration and prevents continuous muscle stimulation.
Muscle action potential → Travels along the sarcolemma (muscle membrane).
Sarcolemma → Action potential moves down into the T-tubules.
T-tubules → Trigger opening of voltage-gated calcium (Ca²⁺) channels in the sarcoplasmic reticulum (SR).
Ca²⁺ (from SR) → Released into the cytosol.
Ca²⁺ → Binds to troponin (on the thin filament).
Troponin binding → Causes tropomyosin to move, exposing myosin-binding sites on actin.
Exposed myosin-binding sites → Myosin heads attach to actin, forming crossbridges.
ATP hydrolysis → Powers myosin heads for power strokes.
Power strokes → Pull thin filaments toward the center of the sarcomere, causing muscle contraction.
Ca²⁺-ATPase pumps → Actively transport Ca²⁺ back into the sarcoplasmic reticulum.
Ca²⁺ reuptake → Lowers cytosolic calcium levels.
Decreased Ca²⁺ levels → Tropomyosin returns to resting position.
Tropomyosin → Blocks myosin-binding sites on actin
Crossbridge cycling stops → Muscle relaxes.
Sarcomere → Returns to resting length.
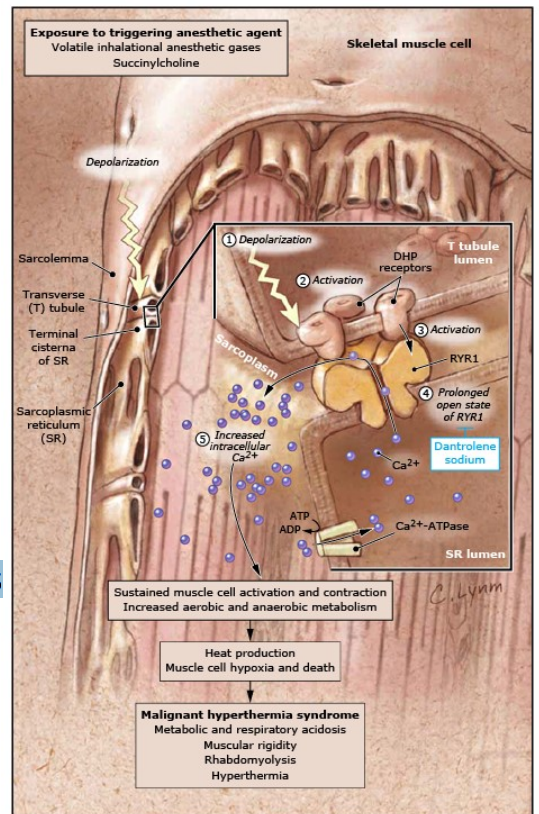
Malignant Hyperthermia
(Tx. Target Ryanodine Receptor)
T tubule/DHP receptor and/or ryanodine receptor/RyR mutations
Ca2+ buildup in sarcoplasm
Sustained muscle contraction
↑CO2 , HR
ATP depletion → anaerobic respiration → acidosis
Further depletion → rhabdomyolysis
Heat from hypermetabolism accumulates
Process of sustained muscle contraction
Myasthenia gravis
Autoimmune disease: antibodies vs AChR
Fatigue, muscle weakness, difficulty with muscle contraction
Improper setup can lead to unnecessary strain on muscles, causing long-term contraction, spasms, and nerve issues. This can result in difficulty contracting muscles due to nerve entrapment. == physical strain and discomfort
Importance of Ergonomics
Nerve Conduction Velocity measures the speed at which electrical impulses travel along a nerve, which helps identify nerve damage or issues.
What does Nerve Conduction Velocity measure?
Elbows should be at 90 degrees.
Arms should be leveled.
Eye level should align with the top of the webcam.
What are some tips for maintaining good occupational health?
A needle is inserted into the nerve, and the electric potential is recorded. This is done to locate the nerve or muscle with the problem. Before doing this test, you would want to cross out carpal tunnel first.
Electromyography
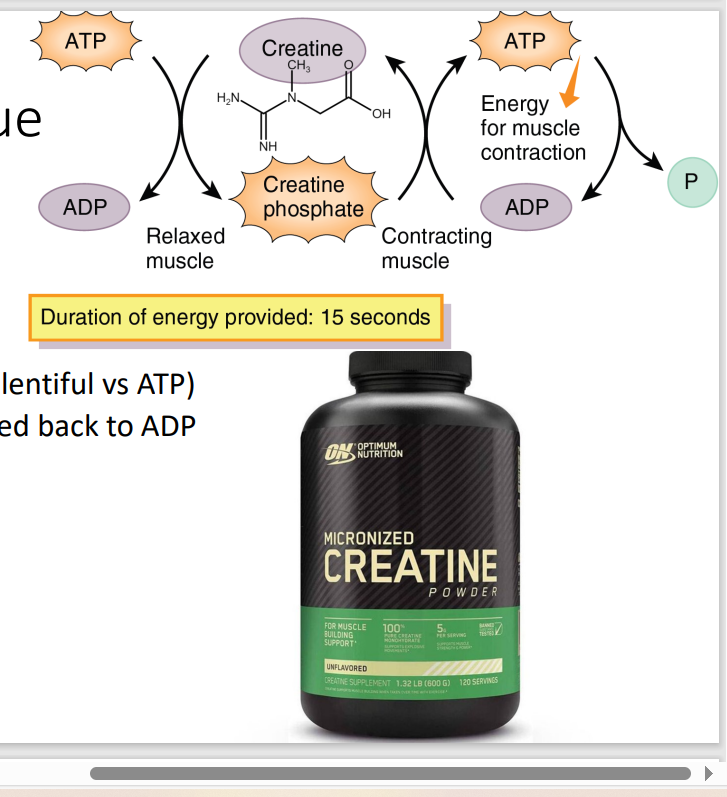
B. The first statement is false. The second statement is true.
I. Creatine kinase (CK) is an enzyme that catalyzes the conversion of creatine and ATP (adenosine triphosphate) into phosphocreatine (creatine phosphate) and ADP (adenosine diphosphate) during contraction.
Creatine is a naturally occurring compound found in muscle tissue, while creatinine is a waste product of creatine metabolism that is excreted in the urine.
I. Creatinine kinase (CK) is an enzyme that catalyzes the conversion of creatine and ATP (adenosine triphosphate) into phosphocreatine (creatine phosphate) and ADP (adenosine diphosphate) during contraction.
II. Creatine is NOT creatinine
A. The first statement is true. The second statement is false.
B. The first statement is false. The second statement is true.
C. Both statements are true.
D. Both statements are false.
energy from the phosphate of ATP faci
Creatine Kinase → get P of ATP and transfer to Creatinine → make Creatinine Phosphate (the energy unit > ATP)
If Creatine Phosphate is the biggest contributor of energy in the muscle, how does get its energy?
During contraction, the phosphate group is transferred from Creatine Phosphate back to ADP, forming ATP.
What happens during muscle contraction regarding creatine?
Indication: Performance Enhancer
S/E: does not dmg kidney
Indication and S/E of Creatine Powder
S/E: Creatine and Drug-induced Liver Injury
Test purity and concentration of Creatine @ DOST-FNRI
don’t consume a lot, herbal and dietary is leading cause to liver failure
S/E and Recommendation for Sub-Optimal Creatine Powder
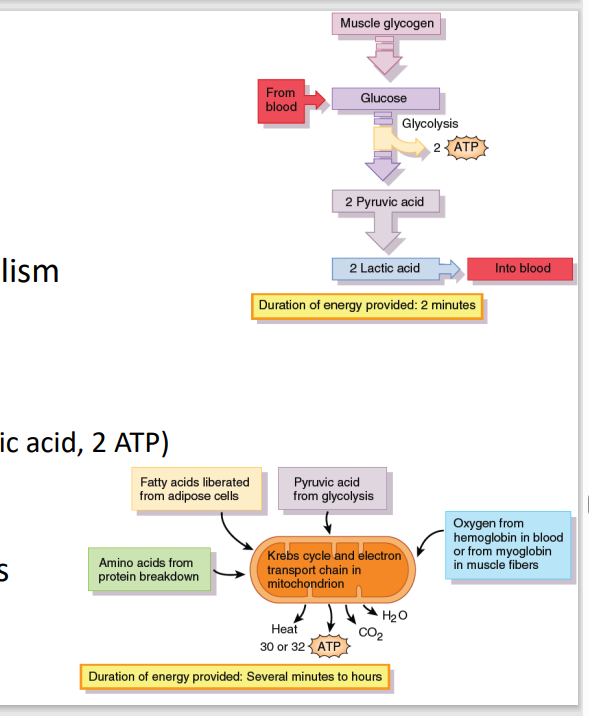
A. Glucose catabolism → pyruvate (2 ATP)
Creatine provides the energy for glucose metabolism converting it into pyruvate and yielding 2 ATP
[Muscle Metabolism: Glycolysis]
Creatine depletion
A. Glucose catabolism → pyruvate (2 ATP)
B. Not enough O2 → anaerobic glycolysis
C. Aerobic glycolysis (Kreb’s cycle, ETC)
B. Not enough O2 → anaerobic glycolysis
Pyruvate converted to lactic acid (2 lactic acid, 2 ATP)
Long term anaerobic glycolysis → Buildup of lactic acid = muscle soreness
[Muscle Metabolism: Glycolysis]
Heavy exercise
A. Glucose catabolism → pyruvate (2 ATP)
B. Not enough O2 → anaerobic glycolysis
C. Aerobic glycolysis (Kreb’s cycle, ETC)
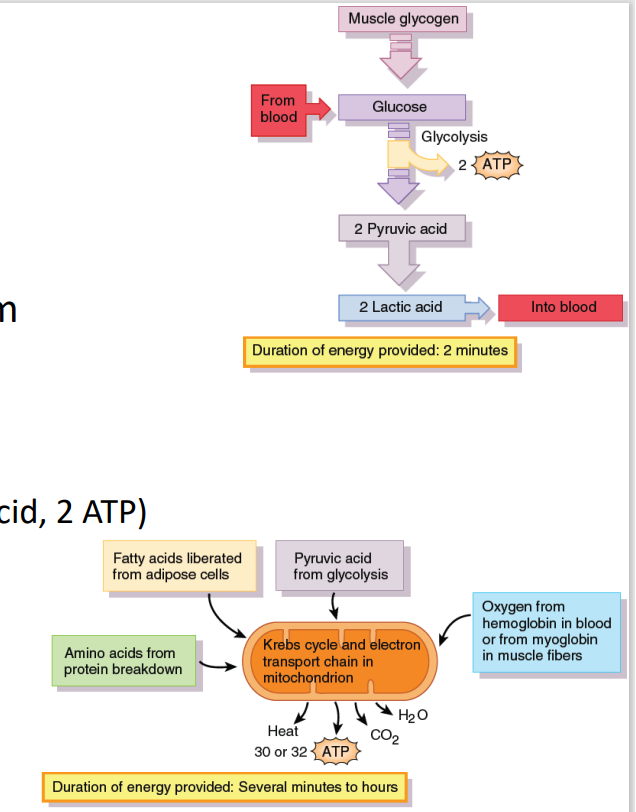
C. Aerobic glycolysis (Kreb’s cycle, ETC)
[Muscle Metabolism: Glycolysis]
If enough O2 from blood hemoglobin /muscle myoglobin
A. Glucose catabolism → pyruvate (2 ATP)
B. Not enough O2 → anaerobic glycolysis
C. Aerobic glycolysis (Kreb’s cycle, ETC)
Since the muscle is damage, the myoglobin would be depleted from the muscle and would be excreted through urine (red-brown urine)
How does Myoglobinuria happen?
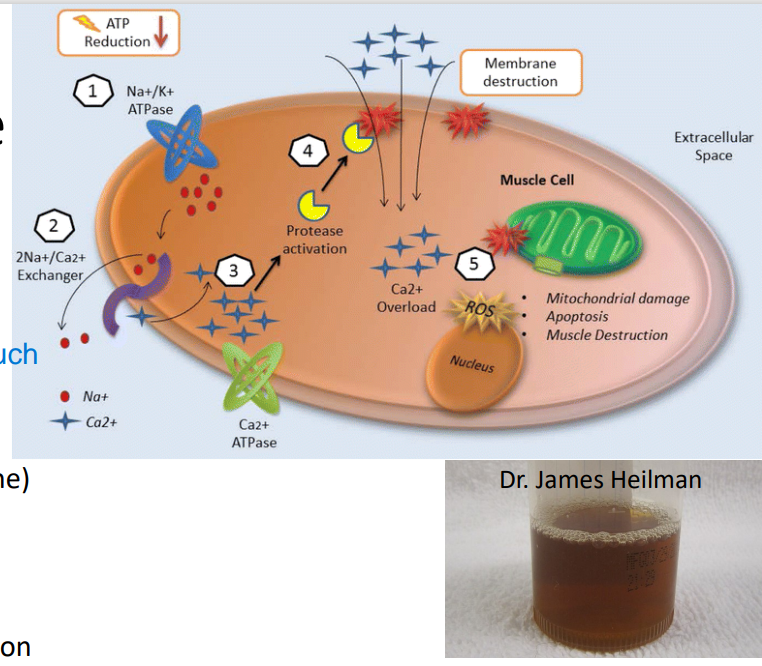
Rhabdomyolysis
Symptoms of Disease:
Myalgia: flu-like (ache, soreness, stiffness, tenderness, cramps)
Myonecrosis; Myoglobinuria (red-brown urine)
Creatine kinase >5x upper limit of normal
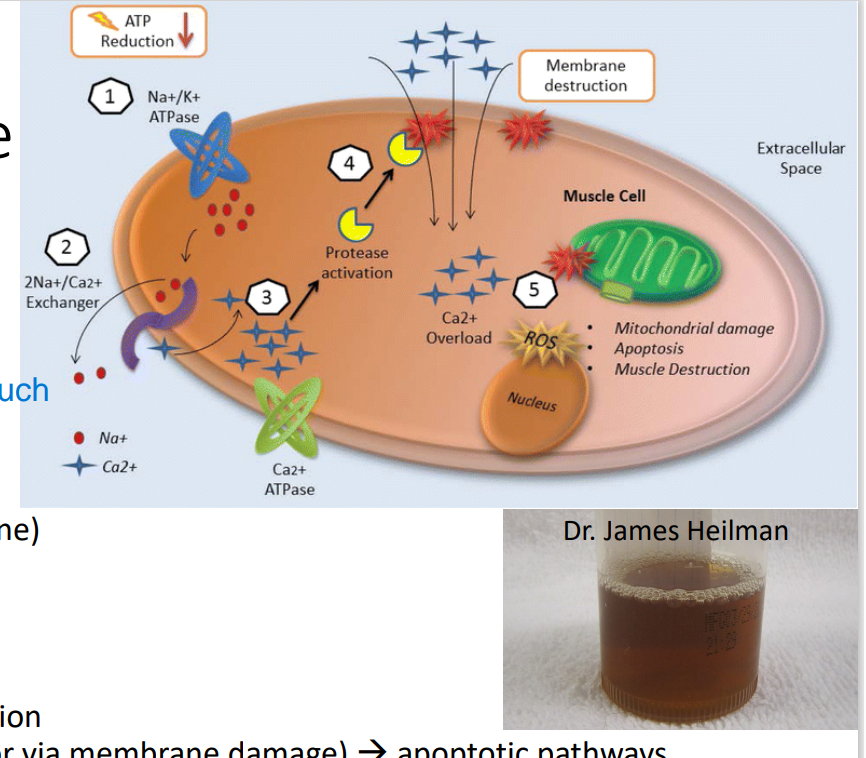
Rhabdomyolysis
Pathophysiology of this Disease:
(adults: trauma, drugs, infections; kids: +exercise)
too much exercise → Energy depletion → Na+ /K+ ATPase dysfunction → ↑Na+ → Na+ /Ca2+ exchanger take over ATPase → ↑Ca 2+ (or via membrane damage) → apoptotic pathways
injure the muscles too much
*Ca2+ enter muscle cell freely if w/ direct injury or trauma