PHAR 380 Module 3: Toxicity Testing
1/177
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
178 Terms
in vitro experiments
experiments that occur outside of a body, such as within a petri dish
in vivo experiments
experiments that occur within a body, such as experiments conducted with animals
in vitro experiments involve...
- cells or tissues maintained or grown in controlled lab conditions to examine toxic properties of compounds
- allows isolation of mechanisms of xenobiotic toxicity without interaction with organisms entire physiological system
4 in vitro models
1. cell culture
2. stem cell culture
3. co-culture of cells
4. tissue culture
cell culture (in vitro models)
mammalian (or other) cells are maintained and propagated under specified conditions outside of organism from which they were derived
3 materials required for cell culture
1. vessel - to contain and nourish cells
2. nutrient media - to feed cells
3. incubator - to house cells at ideal temperature, humidity level, and oxygen/CO2 levels
cell culture divisions
- primary cell culture
- cell line
cell line divisions
- finite
- continuous
primary cell culture
- cells extracted directly from a tissue or organisms
- transferred to culture vessel, where maintained and propagated under specific conditions outside their original environment
cell line
- subcultures of cells from primary cell culture
- primary cells, grown under appropriate conditions, multiply and occupy all space in cell culture dish (confluency)
- cells transferred to new dish, where they can continue to grow and proliferate; these subcultures are the cell lines
finite cell lines
- derived from primary cell culture
- unless modified, capable of limited # of divisions before they senesce, losing ability to proliferate
- can be more indicative of in vivo characteristics than continuous cell lines
- short-lived in culture, and suffer from rapid dedifferentiation
dedifferentiation
when cells regress from specialized function to more simplified state reminiscent of stem cells
continuous cell lines
- derived from primary cell culture, but modified to prevent senescence, allowing immortalized cells to maintain replicative ability and be propagated in culture indefinitely
- transformed, either spontaneously or purposefully altered by viruses or chemicals, and express traits that prevent senescence
- many immortal cell lines cancerous, since cancer cells maintain replicative ability
7 factors to consider when choosing a cell line
1. species
2. experimental purpose
3. finite or continuous
4. normal or transformed
5. feasibility
6. cell characteristics
7. length of study
species (when choosing a cell line)
- which animal makes most sense to use?
- some cell types easier to access
- experiment purpose should dictate whether to use species-specific cultures
experimental purpose (when choosing a cell line)
- what is purpose of experiment?
- i.e., liver toxicant = liver cells, developmental toxicity = cells from embryonic/fetal tissue, signalling pathway specific to humans = human cell line
finite or continuous (when choosing a cell line)
finite:
- may give more options to express correct cellular phenotypes
- and more options of cell type to culture
continuous:
- often easier to propagate and maintain, providing increased consistency in results
- extremely well characterized, which can help predictability for results
normal or transformed (when choosing a cell line)
transformed cell lines
pros:
- continuous and usually have increased growth rate, beneficial when need large quantity of cells for experiment
cons:
- undergone permanent phenotype change that may alter response to toxicant
feasibility (when choosing a cell line)
if using finite line, are sufficient stocks available? access to cell line in lab?
cell characteristics (when choosing a cell line)
- what cell characteristics make most sense for study?
- consider characteristics of cells in relation to research question
- e.g. if wanting to study expression of particular signalling pathway, does in vitro model express required proteins?
length of study (when choosing a cell line)
- how long will cells be exposed to chemical for?
- think of reasonable time frame and if model will function for that long
Question: Deciding on the appropriate cell culture
A hypothetical commonly prescribed drugs for humans is a human teratogen as it causes microglial cell death. Your lab has established that this drug is also a mouse teratogen, however instead of causing microglial cell death in mice, it instead causes satellite cell death. Your lab is interested in investigating the signalling events that cause the cell death, as it relates to your lab's model of teratogenicity. Which is the most appropriate t
Mouse satellite cells
- bc interested in understanding the signalling events that result in the satellite cell death associated with drug exposure in mice
advantage of using cell culture in toxicology
consistency and reproducibility of results
4 experiments commonly conducted using cell cultures
- cell death assay
- proliferation assay
- phenotypic changes
- mechanistic study
assays are an investigative lab technique used to do what
quantitatively or qualitatively assess a particular entry
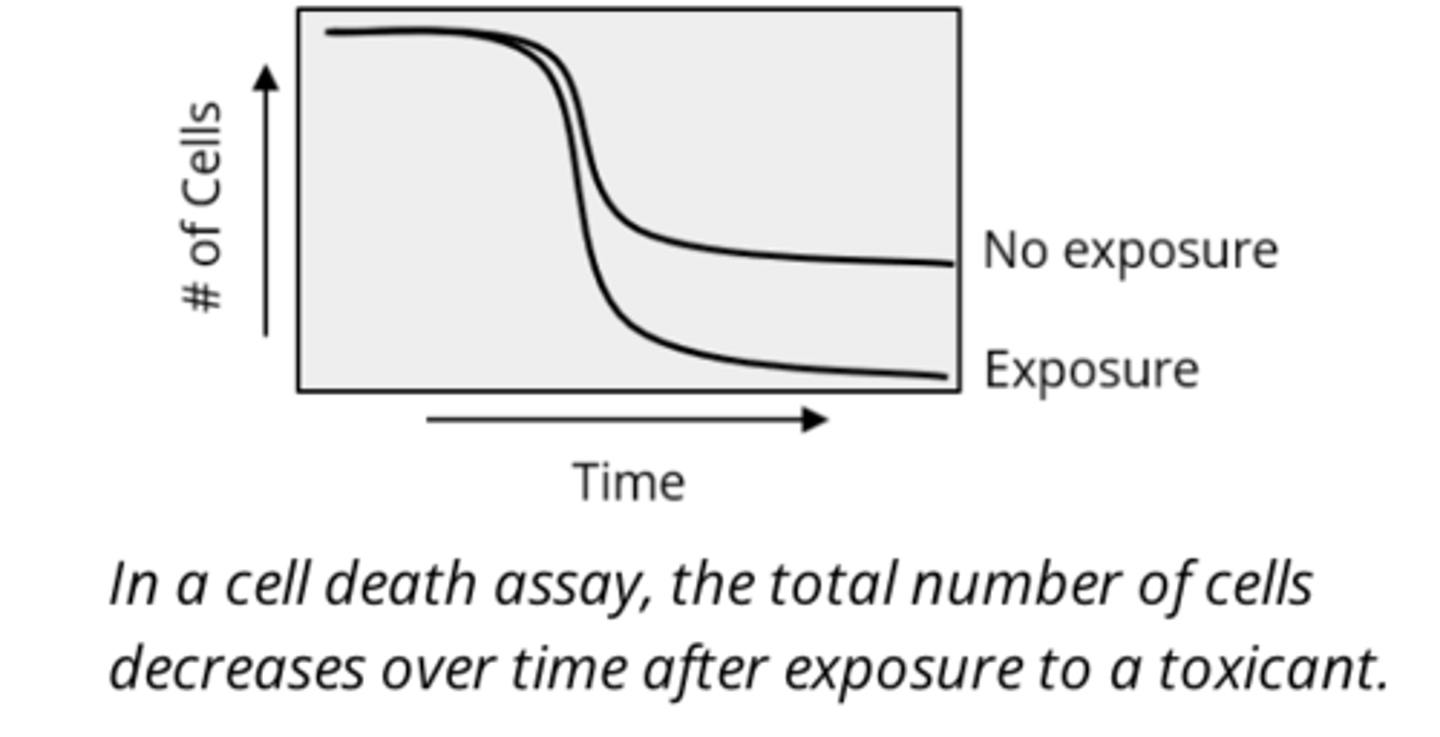
cell death assay (experimental applications of cell cultures)
assess how exposure to a chemical affects the extent or rate of cell death in a particular cell type
proliferation assay (experimental applications of cell cultures)
- aka differentiation assay
- assess whether chemical exposure affects ability of cell type to proliferate or differentiate

phenotypic changes (experimental applications of cell cultures)
assess morphological or phenotypic changes to cell populations

mechanistic study (experimental applications of cell cultures)
- designed to understand a process' mechanism of action
what 3 things could you assess in a mechanistic study?
if chemical exposure causes:
- mitochondrial dysfunction
- cell stress
- alterations in gene/protein or cellular signalling
what do in vitro mutagenicity tests detect?
whether a toxicant causes mutations in cellular DNA
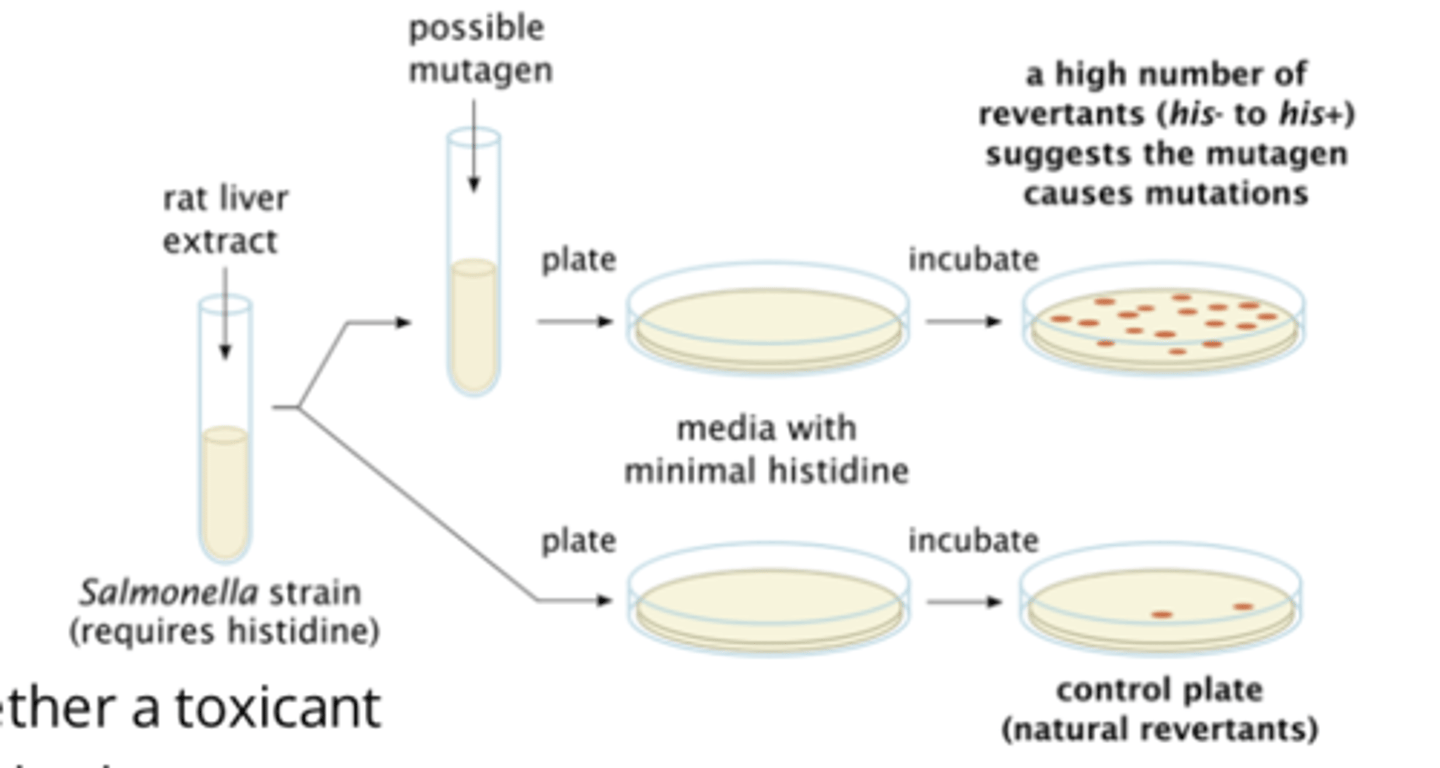
give an example of in vitro mutagenicity test, describe this test
- Ames test
- bac cells unable to produce His (His-), required for growth, cultured in combination with toxicant
- mutation that infers ability to produce His (His+), caused by chemical exposure, allows bac to grow in absence of His, indicating pos test
what is a substantial benefit of in vitro cell culture models
models have ability to be transfected
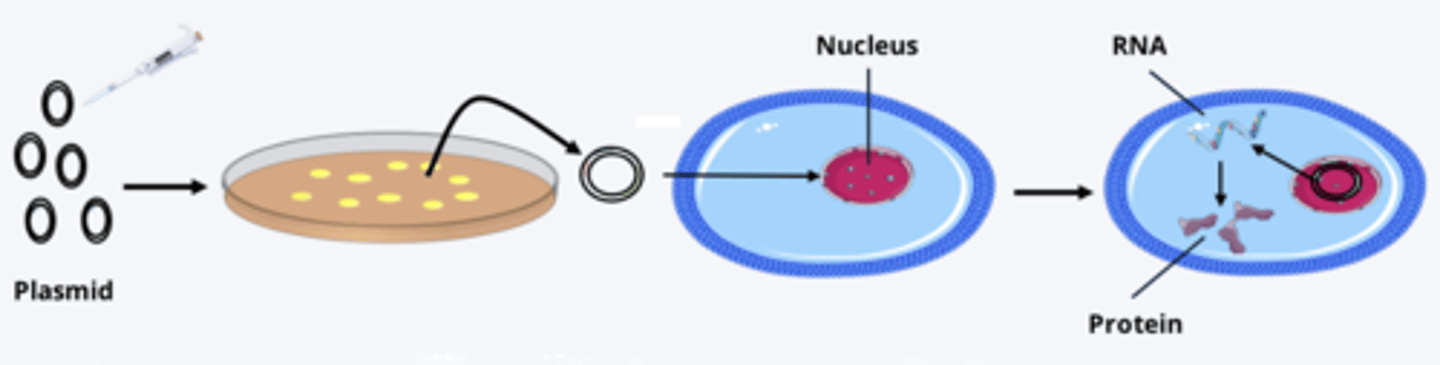
transfection (cell culture)
- process of inserting DNA fragment amplified by PCR or another method (i.e. molecular cloning) into suitable vector (such as plasmid) to introduce that DNA into a cell
- not all cell culture types amenable to transfection
- process allows cells to express gene product they wouldn't otherwise express
- may lead to production of proteins, reporters, inhibitors, and other gene products to provide insight on toxicant's mechanism of action
process of transfection
- plasmid of interest collected and applied to cells
- cell membrane permeability increased chemically or electrically, allowing plasmids to enter cell and nucleus
- once inside nucleus, cell expresses gene product of plasmid, often a protein
limitations of cell culture experiments
- lack of predictive ability
- bc cells cultured out of in vivo environment, no guarantee that cell culture study results predictive of in vivo results
what is a major reason cell cultures may lack predictive ability?
- bc metabolic capabilities of isolated cells aren't reflective of metabolic capabilities of intact organisms
- metabolism changes activity of toxicant profoundly
- if cell culture doesn't have enzymes to metabolize certain chemical, effects may be misrepresented and not consistent with in vivo data
stem cells
cells within the body that are capable of building any tissue type that exists within the body
2 unique features of stem cells
1. able to differentiate into a number of diff specialized cell types within the body
2. able to renew themselves indefinitely
2 ways stem cells are defined
1. developmental stage of animal derived from
2. biological characteristics based on ability to differentiate, divided into pluripotent or multipotent
pluripotent stem cells
can differentiate into all cell types within body, across all germ lines (i.e., endoderm, mesoderm, AND ectoderm)
multipotent stem cells
can differentiate into any cell type within one of the germ lines (i.e., endoderm, mesoderm, OR ectoderm)
why are stem cell cultures a useful in vitro model
consistent with in vivo data, increasing predictive ability over other types of cell culture
3 types of stem cells applicable to toxicology
- embryonic
- adult
- induced pluripotent
embryonic stem cells harvested from what, and defined how
embryo less than 5 days old, and are pluripotent
adult stem cells harvested from what, causes them to be what
adult tissue (bone marrow, skin, cord blood, brain), so limited in type of cells they can differentiate into compared to embryonic stem cells
induced pluripotent stem cells harvested from what, and are induced to what
- somatic cells
- state in which can give rise to number of diff cell types via genetic transformation; also pluripotent
which stem cells are ethically considered the most useful in research? why?
induced pluripotent stem cells
- don't have ethical constraints of embryonic stem cells, but do have pluripotent capabilities, unlike adult stem cells
advantages of using stem cells
- don't lose replicative ability, therefore don't require genetic manipulation
- allow assessment of developmental toxicity in vitro
- toxicity tests have more consistency with in vivo data compared to using conventional cell types
disadvantages of using stem cells
- technically difficult to culture & lack protocols on how to do so
- aren't well characterized
co-culture of cells (in vitro models)
- study interactions between cell populations and fundamental to cell-cell interaction studies
- cell culture in which 2+ diff cell populations grown with contact btwn them
2 co-culture models showing promise
- organotypic model
- organ-on-a-chip
organotypic model (co-culture of cells)
- 2+ cell types from complex tissue or organ cultured together to mimic in vitro tissue
- employs 3D culturing of cells - cells able to interact in more natural way, including formation of extracellular matrix
give an example of an organotypic model you could use if you were interested in dermal toxicity of a particular toxicant
- culture keratinocytes and fibroblasts together since both found naturally in skin and may better mimic in vivo environment of the 2 cell types
- may better reflect possible toxicity seen from dermal toxicant, than either cell type cultured as monoculture
organ-on-a-chip (co-culture of cells)
- cells cultured on a specialized microchip to recapitulate microenvironment of human organ
- similar to organotypic model, but cells grown in microenvironment that better reflects organ system of interest
if a researcher was interested in lung toxicity, organ-on-a-chip techniques would allow for...
culture of cells relevant to lung, along with physiological processes, such as air exposure and stretch force of breathing, imposed on cells to better reflect in vivo environment
what is a potential future direction for organ-on-a-chip technology?
- multiple organs-on-a-chip
- device, w/ each compartment housing specific organ model connected to other chambers via artificial vessels
- could recreate organ connections observed in human body
describe tissue culture (in vitro model)
culturing of entire tissues and organs has been employed for longer than co-culture models, and can produce similar experimental results
culturing of entire tissues and organs has been employed for longer than ____________ ______________, and can...
- co-culture models
- produce similar experimental results
in regards to tissue cultures, numerous organ types have been cultured, either as ___________ or _________ ___________, such as the...
slices or whole organs, such as lung, liver, kidney, skin
describe an embryo culture (mouse or rat) which is an example of a tissue culture
- whole embryos (still in yolk sac) can be removed from uterus and cultured for short period of time
- provide unique mechanistic information to researcher, beyond traditional in vitro or in vivo approaches
what are 2 benefits of the embryonic culture approach
1. effects of toxicant on fetus can be determined independently of effects on mother
2. can culture embryonic organs separately to isolate effects of toxicant exposure on development of specific embryonic structure
Question: Choosing a Cell Culture Model
You hypothesize that a toxicant of interest affects a family of proteins present in neurons, but this has not been demonstrated experimentally. Your lab has access to many diff types of animals as well as popular continuous cell lines derived from human cells.
What in vitro model would you consider using?
Neurons are the cell type in question, and using primary human neurons would not be feasible. Expressing the proteins in a popular continuous cell line derived from human cells, such as human embryonic kidney cells or HeLa cells, could perhaps work.
However, likely the only similarity between these cell lines and the cells of interest is the fact that they are derived from humans. Kidney and cervical cells are unlikely to portray the signalling cascades of interest in a manner that is relevant to your research question. Therefore, isolating neurons from animals, such as mice, may be the best option as you have access to these animals and bc neurons, even from other mammals, are widely used bc they are seen as physiologically similar to human neurons in many ways.
HeLa cells
- oldest and most commonly used immortal cell line in scientific research
- originates from cervical cancer cells
Question: Choosing an in vitro model
A researcher is studying mechanism of toxicity of lung carcinogen and uses tissue culture of human liver slices as an experimental model.
What is the most plausible reason for this researchers choice?
• Human liver slices easily attained
• Tissue culture is more representative of in vivo scenario than cell culture
• Liver slices more easily cultured than liver cell lines
• Human liver slices well characterized and findings will be reproducible
• Tissue culture is more representative of in vivo scenario than cell culture
what is important to consider when conducting experimental research on animals
- must be conducted in ethical manner, according to strict guidelines
- effort across sciences to reduce, replace, and refine animal model use, to ensure used thoughtfully and only when necessary
- before approved by ethics committees, alternative experimental designs should be considered
what types of animals models are useful when initially testing the toxicity of a new chemical?
- animal that hasn't been genetically manipulated or induced to have certain disease characteristics
- afterwards, assessing toxicity in animal model that represents exposure pop (i.e. a disease model) could be appropriate
how is the importance of choosing an appropriate animal model highlighted when selecting an appropriate species for developmental toxicology experiments?
diff types of placentas among mammal species, so picking animal model with placental type similar to humans important to mimic toxicant handling by human placenta
what is the most appropriate reason for the value of animal model in the field: developmental toxicology
consideration of maternal and fetal contributions to toxic response
what is the most appropriate reason for the value of animal model in the field: cardiac toxicology
compensatory mechanisms that could kick in following a toxic response
what is the most appropriate reason for the value of animal model in the field: liver toxicology
changes in toxicant metabolism that would accompany this type of toxicity
what is the most appropriate reason for the value of animal model in the field: nervous system toxicology
behavioural changes associated with toxicant exposure
what 4 research-level factors must be considered when selecting an animal model (in vivo)
- existing knowledge
- statistics
- generalizability
- logistics
existing knowledge (research-level factors)
- consider existing body of research in this area
- important for seeing how your research question fills in knowledge gaps
- helps you consider typical animal used in this research (may not want to use it tho), and narrow down acceptable/useful species and genetic variations
describe existing knowledge related to mice as a model organism for toxicological studies
- extensive knowledge base exists
- from literature, can identify diff strains of mice and experimental methods that can be used
- allows for consistency and comparability across studies
statistics (research-level factors)
- consider statistical power necessary to provide significant results
- often requires consult with biostatistician
- if large sample size needed to assess variable of interest, may influence type of animal selected
statistical power
the likelihood that a study will detect an effect when there's an effect there to be detected. a larger sample size will increase statistical power
describe statistics related to mice as a model organism for toxicological studies
mice useful bc widely available and can feasibly provide large enough samples to produce statistical significance
generalizability (research-level factors)
- consider if info you're gaining is generalizable (transferable) to species of interest (humans)
- if learn something about toxicity of chemical in animal, want to understand what that might mean for humans
describe generalizability related to mice as a model organism for toxicological studies
- genomic studies have shown genetic homologies between mice and humans
- phylogenetic relatedness and physiological similarity to humans, makes them generalizable
logistics (research-level factors)
includes factors that influence feasibility such as cost, animal availability, or animal housing availability
describe logistics related to mice as a model organism for toxicological studies
mice easily maintained and bred in lab setting, making them very feasible
what are 4 animal-specific factors that must be considered when selecting an animal model (in vivo)
- genetic strain
- natural vs. experimental
- biological properties
- sex and lifespan
genetic strain (animal-specific factors)
- diff strains possess diff qualities based on genetics
- consider whether uniformity of animals (i.e. inbred strain) may be beneficial to reduce interplay of genetic variability, or if strain w/ genetic variation would be more useful
- genetic makeup could impact generalizability
natural vs. experimental (animal-specific factors)
- more feasible to have experimentally-produced or natural?
- ecological repercussions to using a natural pop?
- animal strains typically purchased from highly regulated labs that breed specific animal strains
- but sometimes necessary to use a natural/wild pop (e.g. captured fish) to evaluate environmental toxicity
biological properties (animal-specific factors)
- want species that possess similar biology to species and mechanism of interest
- e.g. if interested in specific metabolism process, want animal that undergoes similar metabolic process as humans
sex and lifespan (animal-specific factors)
- when studying aging, might not want species with long life span
- some toxicants can have diff effects on male or female-specific biological processes
describe sex and lifespan related to mice as a model organism for toxicological studies
- lab mice have average lifespan of 3-4 years, making it poss to study aging
- also have early sexual maturity and ability to produce frequent and large litters
how can animal models be used to determine toxicity
- exposing animals to a chemical for a short?
- or long period of time?
- short-term exposure, then look for signs of toxicity/mortality, to identify values such as TD50 and LD50
- long-term exposure to identify pathological effects of chemical exposure like tumorigenesis, then extrapolate to determine toxicity risk in humans
what is an example of an initial test used to determine toxicity of unknown susbtance? (methods of toxicity testing in vivo)
acute oral testing
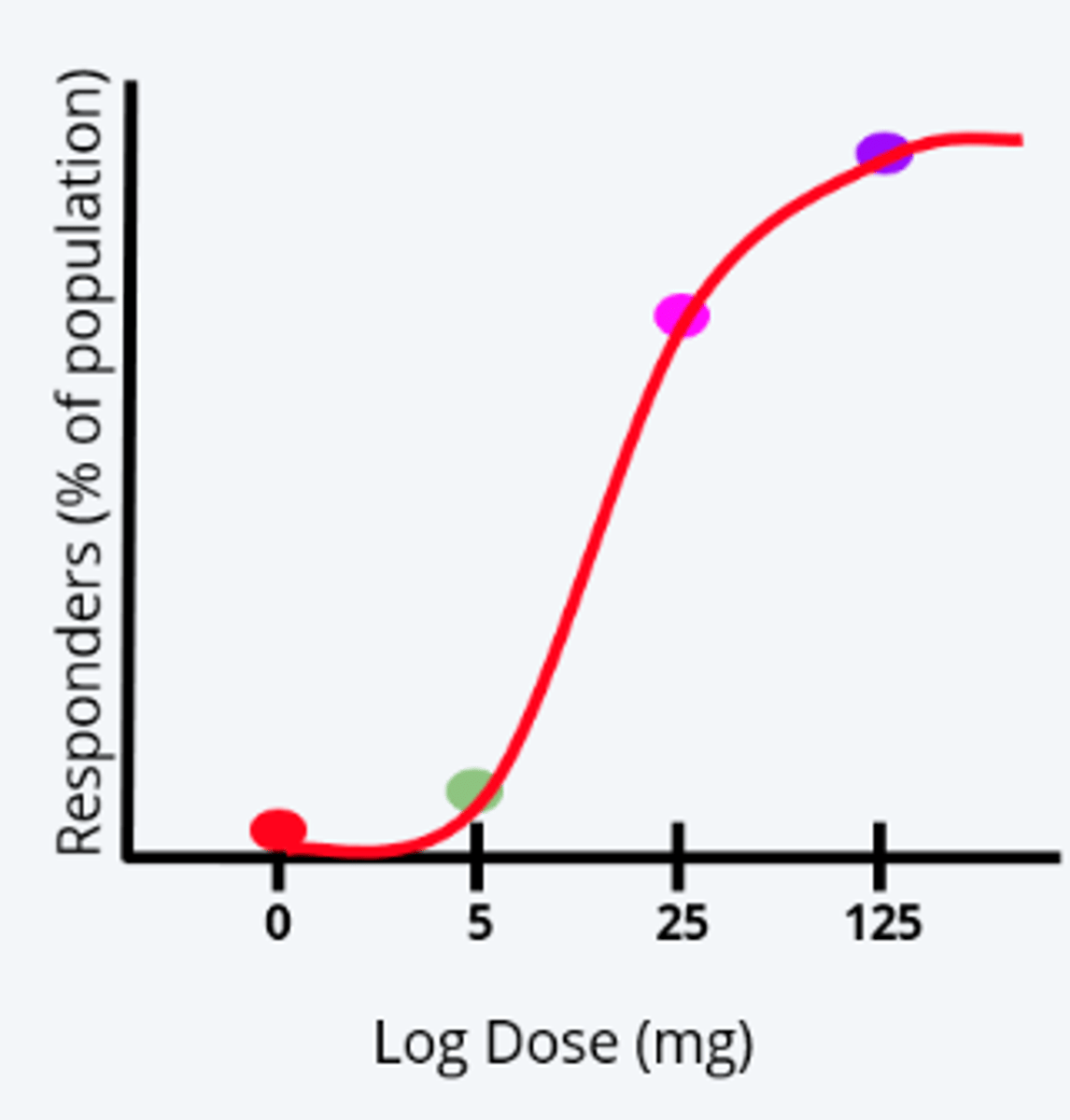
what are the 4 components of acute oral testing that can be used to establish a dose-response curve
1. experimental design
2. dose
3. wait 24h
4. measure & record
how acute oral testing can be used to establish a dose-response curve: 1. experimental design
- start with group of animals (at least 5 of each sex per dose)
- will be exposed to differing doses of toxicant

how acute oral testing can be used to establish a dose-response curve: 2. dose
each group of randomly assigned animals given dose of toxicant or vehicle control (0 mg/kg) by oral gavage needle

how acute oral testing can be used to establish a dose-response curve: wait 24h
- for acute toxicity, wait period for toxic response to occur is within 24 hours of administration
- can measure toxic response at multiple time points
how acute oral testing can be used to establish a dose-response curve: measure & record
- through administration of multiple doses, lethality, onset, nature, severity, and reversibility of toxicity can be assessed
- % of pop that experienced toxic response recorded for each dose to create dose-response curve
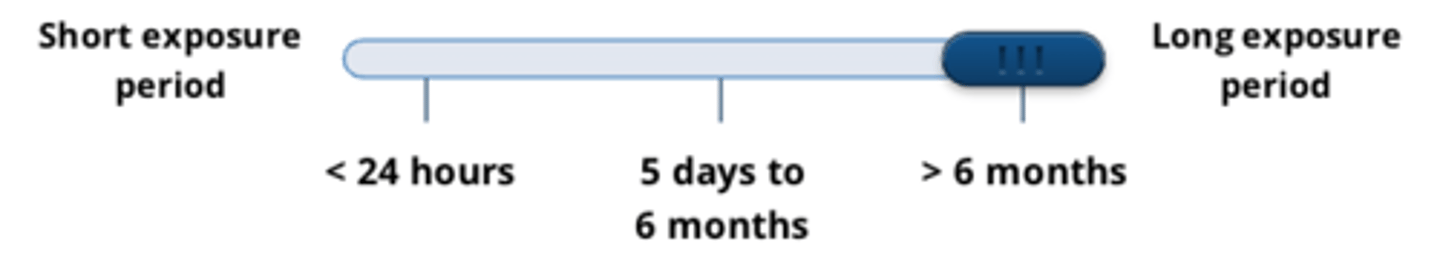
acute tests demonstrate effects of a substance...
after one or multiple exposures in less than 24hr
- short exposure period = <24 hours
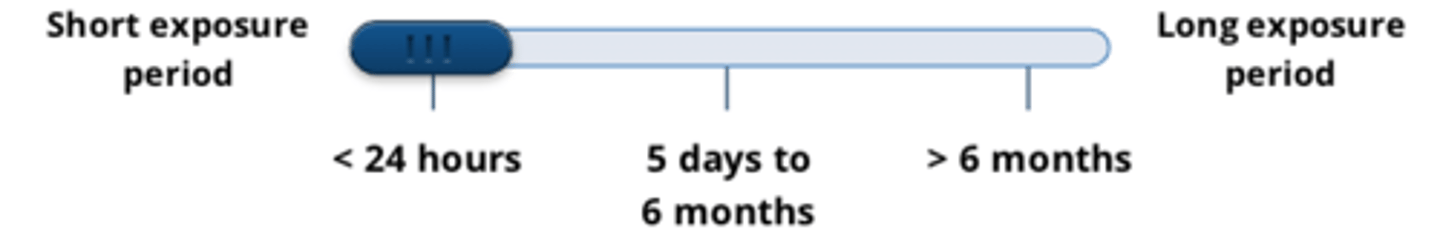
give 3 examples of acute tests
1. eye irritation tests
2. dermal irritation/sensitization tests
3. safety pharmacology tests
sub-chronic tests demonstrate effects of a substance...
after repeated doses, over 5 days to 6 months
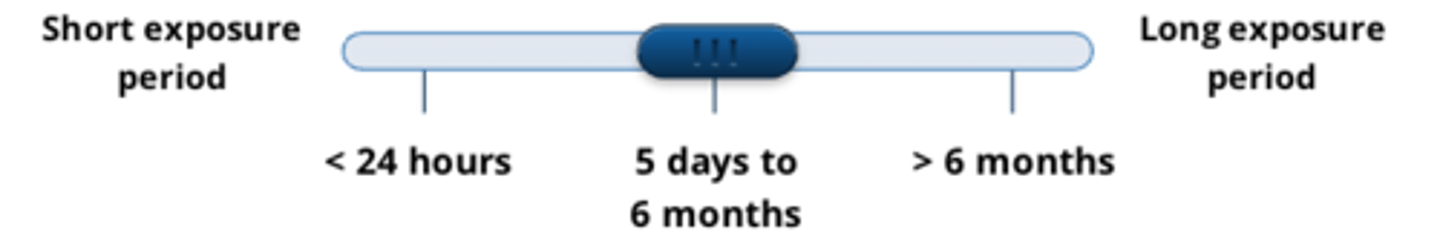
give 4 examples of sub-chronic tests
1. 90-day toxicity tests
2. repeated dose dermal tests
3. reproductive toxicity and teratogenicity tests
4. inhalation tests
chronic tests demonstrate effects of a substance...
after long term exposure to toxicant over period of more than 6 months
- long exposure period = >6months