IMF
1/83
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
84 Terms
fundamental difference between states of matter
distance b/w partciples
gas
total disorder
much empty space
liquid
disorder
partciles/clusters free to move relative to eachother
crystaline solid
ordered arrangement
partciles are essentially in fixed positions
particles close together
condense phases
liquids and solids
particles are close together
state that a substance is in at a particular temperature and pressure depends on
kinetic energy of the particles
strength of attraction b/w the particles
WHY do KE and IMFS affect phase
IMFS stick molecules together
Kinetic Energy Pulls them apart
Solids -IMFS win
Liquid - Tie
Gas- KE wins
IMFS
attractions b/w molecules are not nearly as strong as intramolecular attractions
BUT can control physical properties
melting+boiling point
vapor pressures
viscosities
Dipole
Hydrogen Bonding
London Dispersion Forces
Ion Dipole
important in solutions of ions
make it possible for ionic substances to dissolve in polar solvents
Dipole
molecules that have permanent dipoles attracted to eachother
positive end of one is attracted to the end of the other and vice-versa
these forces are only important when the molecules are close to each other
the more polar the molecule
the higher is its boling point
LDF
attractions between an instantaneous dipole and an induced dipole.
These forces are present in all molecules whether they are polar or nonpolar.
Instantaneous Dipole:
A temporary dipole in a molecule caused by random electron movement.
Induced Dipole:
A dipole created in a molecule by the presence of a nearby instantaneous dipole.
polarizability
refers to the tendency of molecules to generate induced electric dipole moments when subjected to an electric field
Factors affecting LDFS
long, skinny molecules - stronger LDFS
short, fat molecules - weaker LDFS
relationships b/w dispersion forces and increase # of electrons
LDFS increase w/ increased number of electrons because larger atoms have larger electron clouds which are easier to polarize
which have a greater effect - dipole or dispersion
if 2 molecules are comparable shape/size dipole is dominating
if 1 molecules is larger than other - dispersion forces determine physical properties
Hydrogen Bonding
strong type of intermolecular force that occurs when a hydrogen atom bonded to a highly electronegative atom
N,O,F bonded DIRECTLY to H
hydrogen nucleus is highly exposed
Hydrogen Bonding cont.
Molecules can be H-bond donors or acceptors or both
A lone pair on FON can accept H-Bond
the hydrogen that is a donor has to be bonded to FON
Summarizing IMFS
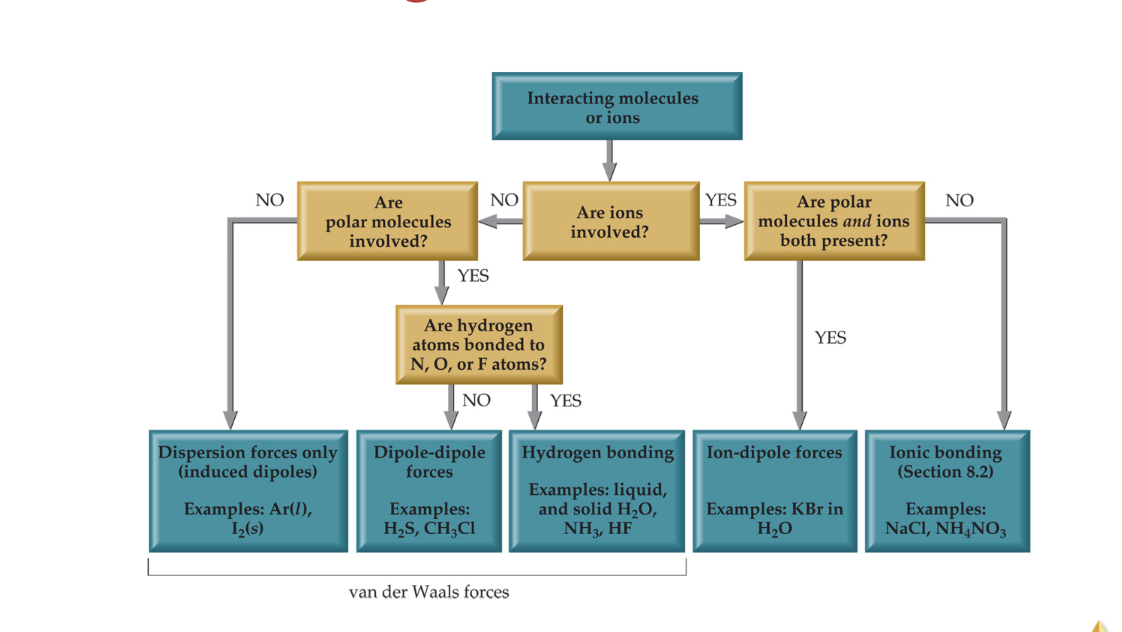
Viscosity
resistance of a liquid to flow
increases with stronger IMFS and decreases with higher temperature
Surface Tension
net inward force experience by the molecules on the surface of a liquid
resistance of a liquid to an increase in its surface area
large IMFS= high surface tension
polar molecules = high surface tension
Chromatography
is used to separate mixtures of substances into their components.
Phases
mobile
stationary
How do components separate?
Different rates due to varying affinities for mobile/stationary phases.
Stationary and mobile phases in paper chromatography?
Stationary = paper, Mobile = solvent (water, ethanol, hexane)
Affinity : mobile vs stationary
High mobile phase affinity → ?
Moves far on chromatogram.
High stationary phase affinity → ?
Moves slowly; stays near start.
Column chromatography principle?
Components move through column at different speeds depending on affinity for stationary phase.
Phases are determine by three things
Kinetic Energy
Pressure
Strength of IMFs
Heat of Fusion
Energy needed to change a solid at its melting point to a liquid
Heat of Vaporization
Energy needed to change a liquid at is boiling point to a gas
Flat/horizontal points on phase change graphs
The substance is changing phase (e.g., melting or boiling) at constant temperature.
Heat energy is used to break/form intermolecular bonds instead of increasing kinetic energy.
Melting (fusion): solid → liquid
Boiling (vaporization): liquid → gas
Endothermic Phase Changes
Melting (Fusion): solid → liquid
Vaporization (Boiling/Evaporation): liquid → gas
Sublimation: solid → gas
Exothermic Phase Changes
Freezing (Solidification): liquid → solid
Condensation: gas → liquid
Deposition: gas → solid
Vapor Pressure
Vapor pressure is the pressure exerted by the vapor (gas) particles of a liquid when the liquid and its vapor are in dynamic equilibrium in a closed container.
Temperature and Vapor PRessure
As temperature increases, more molecules have enough energy to escape, so vapor pressure increases
when a liquid and its vapor reach dynamic equilibrium
The rate of evaporation equals the rate of condensation
When does a liquid boil
When its vapor pressure equals the external (atmospheric) pressure
What is the normal boiling point
The temperature at which vapor pressure = 760 torr (1 atm).
Crystalline Solids
Solids with particles arranged in a highly ordered, repeating pattern.
amorphous solids
Solids with no specific or orderly particle arrangement
covalent-network solids
Solids where atoms are bonded by covalent bonds in a continuous network
Very hard, high melting points.
Diamond, SiO₂ (silicon dioxide), SiC (silicon carbide).
allotrope
Different structural forms of the same element (e.g., diamond and graphite are allotropes of carbon).
molecular solids
Solids where molecules are held together by intermolecular forces.
Softer, with lower melting points compared to network solids.
Ice (solid H₂O).
metallic solid
metal cations surrounded by delocalized valence electrons.
What type of bonding holds metallic solids together?
Metallic bonding — attraction between metal cations and delocalized electrons.
metals conduct electricity- Because delocalized electrons move freely throughout the solid.
metals :malleable, ductile,volatile
are malleable and ductile because : Metal ions can slide past each other without breaking the metallic bond
low volatility- Strong metallic bonding keeps atoms tightly bound, making them hard to vaporize.
alloy
A mixture of a metal with another element (metal or nonmetal)
interstitial alloy
Formed when smaller atoms fill spaces between larger metal atoms
More rigid; decreased malleability and ductility.
substitutional alloy
Formed when atoms of similar size substitute for each other in the lattice
Density between component metals; slightly less malleable and less ductile than pure metals
Do alloys conduct electricity
Yes, most alloys remain good conductors.
What is doping?
Adding a small amount of another substance to improve properties (e.g., conductivity).
: What is n-type doping
Replacing an atom with one that has more electrons, adding extra free electrons for conduction
What is p-type doping?
Replacing an atom with one that has fewer electrons, creating “positive holes” that allow electrons to move and conduct.
How does doping affect conductivity?
It increases conductivity by adding free electrons (n-type) or holes (p-type).
Solutions
homogeneous mixtures of two or more pure substances.
In a solution, the solute is dispersed uniformly
throughout the solvent.
How Does a Solution Form?
Solvent molecules attracted to surface ions.
Each ion is surrounded by solvent molecules.
Enthalpy (ΔH) changes with each interaction broken or
formed.
Solvated
a solute, or dissolved substance, has been surrounded by a solvent
if the solvent is water the ions are hydrated
interparticle force in solutions
ion-dipole.
Enthalpy Changes in Solutions
1) Separation of solute
particles.
2) Separation of solvent
particles to make
‘holes’.
3) Formation of new
interactions between
solute and solvent.
Entropy
Dispersal of energy in
the system.
Number of microstates
(arrangements) in the
system (i.e. disorder)
more spread out - higher
less spread out - lower
Dissolution
usually considered a physical change —you
can get back the original solute by evaporating the
solvent.
If you can’t, the substance didn’t dissolve, it reacted.
Saturated Solution
Solvent holds as much
solute as is possible at
that temperature.
Undissolved solid
remains in flask.
Dissolved solute is in
dynamic equilibrium
with solid solute
particles.
Unsaturated Solution
Less than the
maximum amount of
solute for that
temperature is
dissolved in the
solvent.
No solid remains in
flask.
Supersaturated
Solvent holds more solute than is normally
possible at that temperature.
These solutions are unstable; crystallization can
often be stimulated by adding a “seed crystal” or
scratching the side of the flask.
Factors Affecting Solubility
Polar substances tend to
dissolve in polar solvents.
Nonpolar substances tend
to dissolve in nonpolar
solvents.
The stronger the
intermolecular
attractions between
solute and solvent,
the more likely the
solute will dissolve.
Gases in solutions
solubility of gases in water increases with increasing mass.
solubility of liquids and solids with pressure
does not change with pressure
solubility of a gas in a liquid
directly proportional to its pressure.
How does temperature affect the solubility of most solid solutes in liquids
Solubility increases with increasing temperature.
: How does temperature affect gas solubility in liquids?
Solubility decreases as temperature increases.
Why do gases become less soluble at higher temperatures?
: Higher temperature gives gas molecules more kinetic energy, allowing them to escape the solution.
Mass Percentage
Mass % of A =
mass of A in solution/
total mass of solution
× 100%
Volume Percentage
Volume% of A =
(vol of A in solution/
total vol of solution)
× 100%
Mole Fraction
Moles of solute/moles of solute+solvent
Colloids
Suspensions of particles larger than
individual ions or molecules, but too small to
be settled out by gravity.
Tyndall Effect
Colloidal suspensions
can scatter rays of light.
Colloids in Biology
Some molecules have
a polar, hydrophilic
(water-loving) end and
a nonpolar,
hydrophobic
(water-hating) end.
Intermolecular Forces are NOT
bonds. Avoid
using the word bonding when referring to IMFs.
(i.e. don’t say something like “both molecules are
polar so they have strong bonds”)
IMFS are not found in
ionic compounds
what not to use as an explanation for increasing LDFS
increased mass as an explanation for
increasing LDFs. Instead use “more polarizable”
(or more electrons, so it is more polarizable)
single molecule does NOT have
have hydrogen
bonds. H-Bonds are between 2 (or more)
molecules. (Don’t say things like “A water molecule
has hydrogen bonding” or “a water molecule has
hydrogen bonds between the O and H”)
if molecules are of similar size( number of electrons) IMFS rank
LDF < Dipole-dipole < H-bonding
when dissolve in water
Molecules do not ionize or break apart
sugar molecule is still a
sugar molecule when dissolved in water. Ionic
compounds ionize (or dissociate) into their
component ions.
Volatile refers to
vapor pressure
High volatility = high vapor pressure
Low volatility = low vapor pressure
Which substance is more volatile = which
substance has high vapor pressure