Chem 115 Exam 2 - Electron Configuration, Periodic Trends, Bonding
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
light shining on metal
when light shines at metal at specific wavelength an electron is ejected
shells in first row
1
shells in second row
2
shells in third row
3
IE on periodic table
increases to top right corner
electron affinity
increases to top right corner
electronegativitiy
increases to top right corner
atomic radius
increases to bottom left corner
non metal characteristics on periodic table
increases diagonally to top right corner
metal characteristics on periodic table
increases diagonally to bottom left corner
dimagnetic
electrons are only paired in orbitals, they are not attracted by magnetic field
paramagnetic
one or more orbits with unpaired electrons, weakly attracted by magnetic field
n (principle)
size given by row on periodic table
lower = smaller orbit and energy
l (angular)
shape given by s p d and f
s orbitals
far left section on periodic table
2 electrons
l = 0
p orbitals
far right side of periodic table
6 electrons
l = 1
d orbitals
center section of periodic table
10 electrons
l = 2
f orbitals
separate section on periodic table
14 electrons
l = 3
ml (magnetic)
orientation in space or orbitals
corresponds to l
l = 1 : ml = (-1,0,1)
ms (spin)
two directions possible (arrow up or arrow down)
sub level filling order
diagonal starting with s1 → s2 → 2p → 3s
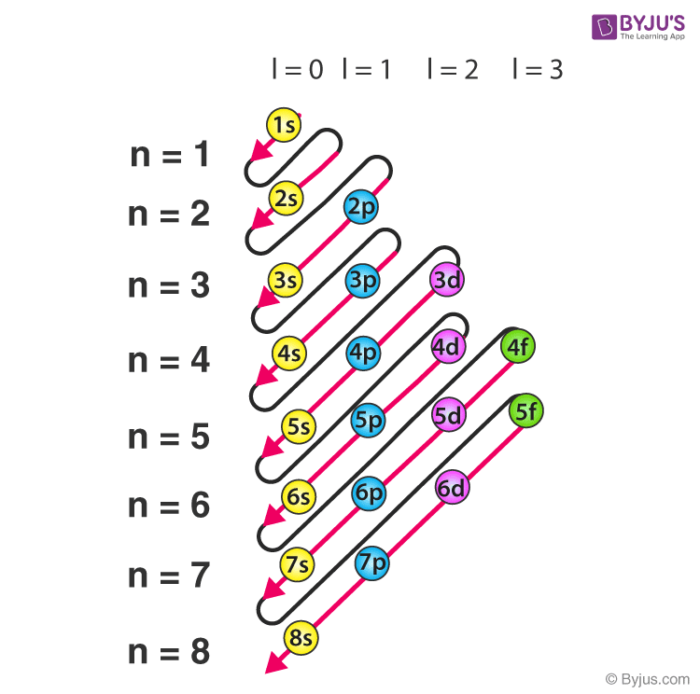
final electron level shows…
row section ^ number in section
metal characteristics
form cations
basic
reductive → electrons gained
high melting point
good conductor
nonmetal characteristics
form anions
acidic
oxidation → electrons lost
low melting point
bad conductors
bond strength
increases up columns
ionic bond characteristics
different electronegativities
between metal and nonmetal
nonpolar
covalent bond characteristics
similar electronegativies
between 2 non metals
polar
bent shape
2 lone pairs and 2 bonds
valance electrons on periodic table
increases left to right skipping center section
lattice energy
given steps work backwards, then total each delta H (MIND THE LEAD COEFFICIENTS)
enthalpy
Hf = Hreactant - Hproduct
lattice energy increases with
small radius
lattice energy decreases with
large radius
stable electron configuration
full or half filled sections
noble gases on periodic table
far right column
isoelectronic
same charge as a noble gas
isoelectronic series
in order from atom increasing charge, to noble gas, to atom decreasing charge
outer electrons
furthest out shell with the highest n number
valance electrons
last set of electrons in diagonal structure
low bond dissociation
low electronegativity
high bond dissociation
high electronegativity