2.2.1 Electron structure
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
shells
electrons arranged around nucleus in principle quantum energy levels
principal quantum numbers (n) used to number energy levels
lower number=closer to nucleus
how many electrons can each principle quantum energy level hold
n=1→ 2
n=2→ 8
n=3→ 18
n=4→32
subshells
principal quantum shells split into subshells:
s holds 2 electrons
p holds 6 electrons
d holds 10 electrons
f (for elements with 57+ electrons) holds 14 electrons
orbitals
subshells contain atomic orbitals
orbitals exist at specific energy levels- electrons only found at these levels, not in-between
each orbital can only hold 2 electrons
s orbital shape
spherical shape
size increases with shell number
p orbital shape
dumbbell shape
every shell has 3 p orbitals except 1st
p-orbitals occupy x,y,z axes- perpendicular
lobes are larger and longer with increasing shell number
filling orbitals
electrons can spin either clockwise or anticlockwise
electrons with same spin repel each other- spin-pair repulsion:
electrons will occupy separate orbitals in same subshell to minimise repulsion
electrons in an orbital must have opposite spin
ground state
most stable electron configuration of an atom which has the lowest amount of energy
4s orbital filled and emptied before 3d orbital because it has lower energy
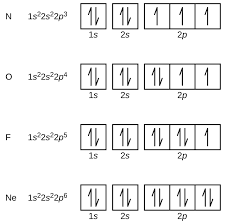
box diagrams
each orbital can hold up to 2 electrons
orbitals are modelled as boxes
an electron is shown as a single headed arrow with either an up or down spin
chromium and copper
elements are more stable if the d shell is partially or fully filled
chromium and copper are one electron off having a fully or partially filled shell, so an electron is promoted to the d shell to complete it
i.e. Cu → [Ar] 4s13d10 Cr → [Ar] 4s13d5
electron configuration of atoms
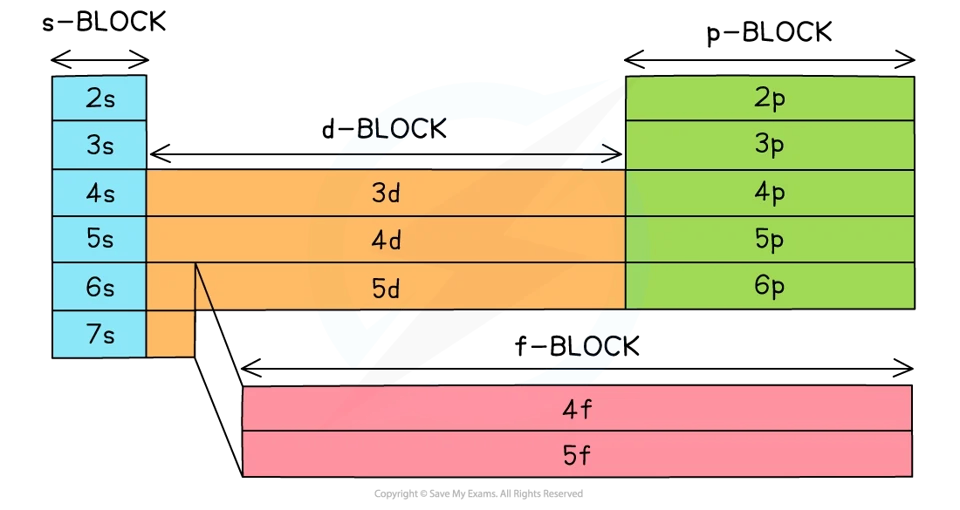
block in which element is in tells us the which orbital the valence electrons are in
period tells us which shell they are in.