M1: proteins practice quiz
1/99
Earn XP
Description and Tags
Chapter 3: https://quizlet.com/91815952/chapter-3-test-bank-flash-cards/
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
100 Terms
Which statement is correct with respect to the amino acid composition of proteins?
Proteins with different functions usually differ significantly in their amino acid composition
For amino acids with neutral R groups, at any pH below the pH of the amino acid, the population of amino acids in solution will have ____.
an overall net positive charge
To determine the isoelectric point of a protein, first establish that a gel:
exhibits a stable pH gradient when ampholytes become distributed in an electric field.
Select all that apply!
Which statement is NOT correct concerning cooperative binding of a ligand to a protein?
It is usually a form of allosteric interaction
It is usually associated with proteins with multiple subunits
It results in a sigmoidal binding curve
It results in a nonlinear Hill plot
Which statement comparing hemoglobin and myoglobin is NOT correct?
Hemoglobin has a stronger binding affinity for O2 than myoglobin does
Which antibody is secreted as a cross-linked pentamer?
IgM
Select all that apply!
Permanent actin-myosin interaction defines the state of rigor mortis that we all want to avoid as long as possible. Actin-myosin interaction during our life ___.
is interrupted when ATP binds to myosin
is an example of protein-ligand interaction
leaves the partipants unchanged
Which statement regarding enzyme activity is correct?
Enzymes bind the transition state better than the reaction products and reduce the activation energy required for the reaction to take place
One of the enzymes involved in glycolysis, aldolase, requires Zn2+ for catalysis. When the enzyme links zinc, it is referred to as the:
apoezyme
____ is an irreversible enzyme regulatory mechanism?
Activation of a zymogen
Both water and glycose include an -OH that can serve as a substrate for a reaction with the terminal phosphate of ATP catalyzed by hexokinase. Glucose, however, is about a million times more reactive as a substrate than water. The BEST explanation is that:
glucose induces a conformational change in hexokinase that brings active-site amino acids into position for catalysis.
After you mix a substrate and enzyme together, there is an initial transient period, the pre-steady state. What happens during the pre-steady state?
ES increases until it reaches a constant level
These data were obtained in a study of an enzyme known to follow Michaelis-Menten kinetics.
Collected data:
V0 | Substrate added | | ||
|---|---|---|---|---|
(μmol/min) | (mmol/L) | | ||
_____________________ | ||||
217 | 0.8 | | ||
325 | 2 | | ||
433 | 4 | | ||
488 | 6 | | ||
647 | 1,000 | | ||
The Km for this enzyme is approximately:
2 mM
Vmax for an enzyme-catalyzed reaction:
is twice the initial rate observed (Vo) when [S] = Km
The amino acid proline is unique because the R group:
is cyclical
Titration of valine by a strong base, for example NaOh, reveals two pK’s (see Table 3-1). Which is the titration reaction occuRring at pK2 (pK2 = 9.62)?
— NH3+ + OH - → - NH2 + H2O
Which component is absolutely necessary for the purification of a protein?
a means of detecting a protein
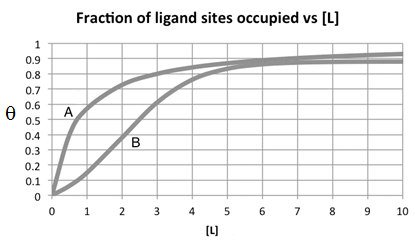
Examine the binding curves for two proteins (A and B) that bind the same ligand. Which statement applies?
The dissociation constant for A is less than that of B
If a developing fetus made beta hemoglobin subunits instead of gamma hemoglobin subunits, what would be MOST likely to occur?
The fetus would not be able to extract O2 from its mother’s blood as effectively
Nerve impulses control muscle contractions by releasing which ion?
Ca2+
Enzymes are potent catalysts because they:
Lower the activation energy for the reactions they catalyze
A protein ______ catalyzes the attachment of phosphoryl groups to specific amino acid residues including ______.
kinase; Thr and Ser
Select all that apply
Enzymes contribute to the reaction rate enhancement via entropy reduction, which may include ____.
Constriction of the substrate in the proper orientation
Aligning the substrates precisely on the enzyme with the help of strategically located groups
Restriction of the relative motion of two substrates
Which statement is false about the saturation effect?
It is responsible for the linear increase of V0 with increase in [S].
We use an enzyme at a 20nM total concentration, and its substrate at 40uM and measure Vo = 9.6 uM/s. Calculate Km, if the catalytic rate constant (kcat) of this enzyme is 600s-1.
10
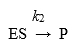
For enzymes in which the slowest (rate-limiting) step is the reaction
Km becomes equivalent to:
the dissociation constant, Kd, for the ES complex
Which type of structure describes the overall three-dimensional folding of a polypeptide?
tertiary structure
Which amino acid has three pKa values?
Cys
In an SDS-PAGE experiment, two markers of molecular weight, 11,000 g/mol and 3,000 g/mol, traveled 5 cm and 9 cm, respectively. Approximately, how far will a fragment of size 7,945 g/mol travel?
6 cm
Which statement about protein-ligand binding is correct?
The larger the Ka, the smaller the Kd (dissociation constant)
In peripheral tissues:
O2 is released
You are working as a scientist in an antibody-design group at a biologies company. Based on your knowledge of antibodies, which region(s) on which antibody type should you target for your design efforts to develop new antigen-binding capabilities in soluble antibodies?
IgG antibodies, variable light and variable heavy chain regions
Tropomyosin and troponin prevent muscle contractions in the absence of Ca2+ because they:
block the myosin head binding sites on actin filaments
Which statement is false about enzyme catalysts?
They increase the equilibrium constant for a reaction, thus favoring product formation
Enzymes:
are proteins (with few exceptions).
Competitive inhibitors:
occupy the active site to exclude the substrate
An enzyme-catalyzed reaction was carried out with the substrate concentration initially a thousand times greater than the Km for that substrate. After 9 minutes, 1% of the substrate had been converted to product, and the amount of product formed in the reaction mixture was 12 μmol. If, in a separate experiment, one-third as much enzyme and twice as much substrate had been combined, how long would it take for the same amount (12 μmol) of product to be formed?
27 mins
The number of substrate molecules converted to product in a given unit of time by a single enzyme molecule at saturation is referred to as the:
turnover number.
The chirality of an amino acid results from the fact that its a carbon _____
is bonded to four different chemical groups
Identify the pair of peptides that are NOT distinguished by tandem mass spectrometry.
VTSPLYANEGK and VTSPIYANEGK
Select all that apply!
When investigating ligand binding, the dissociation constant (Kd) ____.
Equals the free ligand concentration, when half the ligand sites are occupied
Corresponds to the reciprocal of the association constant
Which molecule is homotropic modulator of oxygen binding to hemoglobin?
oxygen
Macrophages bind to the ___ region of IgG antibodies, thereby triggering phagocytosis of an antibody-antigen complexes.
Fc
Thick filaments are made of ____.
myosin
The concept of induced fir refers to the fact that:
Substrate binding may induce a conformational change in the enzyme, which than brings catalytic groups into proper orientation
Which statement about allosteric control of enzymatic activity is false?
Heterotropic allosteric effectors compete with substrate for binding sites
Chymotryspin:
uses the oxygen of a serine side chain as a nucleophile
Which amino acid is NOT capable of using its side chain (R group) to participate in general acid-base catalysis?
Val
Phenyl-methane-sulfonyl-fluoride (PMSF) inactivates serine proteases by binding covalently to the catalytic serine residue at the active site; this enzyme-inhibitor bond is NOT cleaved by the enzyme. This is an example of what kind of inhibition?
irreversible
We use an enzyme at a 10 nM total concentration and measure Vo = 3 uM/s. We also determined that Km = 10 uM and the catalytic rate constant (kcat) of this enzyme is 600 s-1. What was the [S] used in this experiment?
10
The Lineweaver-Burk plot is used to:
solve, graphically, for the rate of an enzymatic reaction at infinite substrate concentration
What are the name and abbreviations of the amino acid shown?
Asparagine; asn; N
Glutamic acid is an amino acid with pKa values of 2.19, 4.5, and 9.67, a-ketoglutarate is a bivalent organic acid. What is the approximate charge
1/2
The interactions of ligands with proteins:
are usually transient
Sickle cell anemia is caused by a single mutation in the hemoglobin protein from a Glu to a Val, which results in which alteration in the hemoglobin subunits?
fewer negative charges and formation of a hydrophobic contact point
Which statement is false?
T lymphocytes produce immunoglobulins
Which protein undergoes a conformational change upon interacting with Ca2+, leading to simulation of muscle contraction?
troponin
The role of an enzyme in an enzyme-catalyzed reaction is to:
Increase the rate at which substrate is converted into product
The role of the metal ion (Mg2+) in catalysis by enolase is to:
stabilize an intermediate during general base catalysis
Phosphorylation of enzyme:
Generally occurs on Ser, Thr, and/or Tyr side chains
The total enzyme concentration is [Et] = ____ nM, if [S] = 6 mM, Vo = 480 nM/min, Km = 4uM, and the catalytic rate constant (kcat) of the enzyme is 20. min-1
24
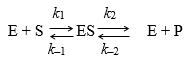
For the simplified representation of an enzyme-catalyzed reaction shown, the statement “ES is in steady-state” means that:
k1[E][S] = k-1[ES] + k2 [ES]
Which is the dominant form of glycine at its isoelectric point?
NH3+—CH2—COO-
The term “specific activity” differs from the term “activity” in that specific activity ___.
is the activity (enzyme units) in a milligram of protein.
What description is associated with immunoglobulin G?
More abundant immunoglobulin
During muscle contraction, hydrolysis of ATP results in a change in the:
conformation of myosin.
Which statement about enzyme-catalyzed reactions is false?
The activation energy for the catalyzed reactions is the same as for the uncatalyzed reaction, but the equillbrium constant is more favorable in th enzyme-catalyzed reaction
What is the advantage of regulatory enzymes using several regulatory mechanisms?
Cells can catalyze only the reactions that are needed at a given moment.
An enzyme that can convert glucose into fructose is a member of which class of enzymes?
isomerases
In what ways does an uncompetitive inhibitor bind to an enzyme?
It reversibly binds to the enzyme-substrate but does bind to the free enzyme
Why can only some amino acids be used to measure protein concentration based on absorption of UV light?
Only some amino acids are aromatic.
Regarding the models of cooperativity:
T state is low affinity and R state is high affinity
Select all that apply!
X-ray analysis has revealed 2 conformations of hemoglobin: the R state and the T state
Oxygen stabilizes R state
when oxygen binds the T state hemoglobin, then the T state hemoglobin will transition into R state
The lack of oxygen result in the T state; thus, this is the dominant conformation in deoxyhemoglobin
Polyclonal antibodies:
are used as analytic reagents in Western blot assays
During muscle contraction, hydrolysis of ATP results in a change in the:
conformation of myosin
Enzymes are potent catalysts because they:
lower the activation energy for the reaction they catalyze
Chymotrypsin:
uses the oxygen of a serine side chain as a nucleophile
An enzyme accepts H+ from hydronium and transfers it to an amine group of the substrate. The result is an increase in the rate of release of the product. This is an example of:
general acid catalysts
What is the definition of Km, the Michaelis constant?
the concentration of substrate at which the enzyme is operating at half its maximal velocity
All of the amino acids that are found in proteins, except for proline, contain a(n) _____ group.
amino
A major advance in the application of mass spectrometry to macromolecules came with the development of techniques to overcome which problem?
Mass spectrometric analysis involved molecules in the gas phase
Select all that applies!
The binding site of a protein is complementary to a specific ligand due to which characteristic of the binding site?
its charge
its size
its shape
its hydrophobicity
The Fab regions of an antibody are made of which protein chain?
both a light chain and a heavy chain containing variable and constant regions
The intestinal enzyme is produce initially as a ____, which requires ____ for activation.
zymogen; irreversible proteolytic cleavage
If a first-order reaction for the unimolar reaction S → P has a rate constant k = 0.05s-1, how is this interpreted qualitatively?
5% of the available S will be converted to P in 1 second
In a mixture of five proteins listed, which should elute second in size-exclusion (gel-filtration) chromatography?
immunoglobulin G, Mr = 145,000
Which molecule binds MOST strongly to the heme iron?
CO
Which statement is false regarding the enolase reaction?
Two Mg2+ ions act as coenzymes in this reaction.
he benefit of measuring the initial rate of reaction Vo is that at the beginning of a reaction ______ as long as the substrate concentration, [S], is much higher than the enzyme concentration, [E].
changes in [S] are negligible, so [S] can be treated as a constant
For a specific enzyme/substrate system, which condition will ALWAYS result in an increase in V0 (assuming nothing else changes)?
A decrease in Km and an increase in Vmax
The following data set indicates that Km = ____ mM and Vmax = ___ uM/min.
Data set
S (mM) | Vo (μM/min) |
|---|---|
0.0025 | 28 |
0.0040 | 40. |
0.010 | 70. |
0.020 | 95 |
0.040 | 112 |
0.10 | 128 |
2.0 | 139 |
10 | 140 |
0.010
70
All of the 20 common amino acids contain an R group that is attached to the:
a carbon
Which amino acid would MOST likely be found in the interior of a globular protein?
Ala
When the partial pressure of oxygen is equal to the P50 of myoglobin, what is the value of Y?
0.50
Myoglobin and the subunits of hemoglobin have:
very similar tertiary structures, but different primary structures.
Which description is associated with immunoglobulin G?
most abundant immunoglobulin
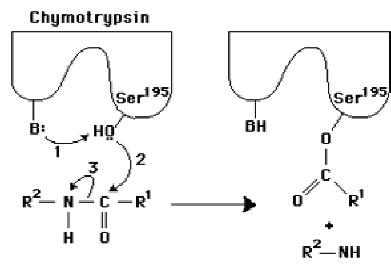
In this diagram of the first step in the reaction catalyzed by the protease chymotrypsin, the process of general base catalysis is illustrated by the number ____, and the process of covalent catalysis is illustrated by the number ____.
1;2
What is the free-energy starting point for a reverse reaction designated as?
ground states
Which statement is false for enzymes where dissociation of the product (k3) is rare-limiting?
Following the pre-steady state phase, the observed rate of product formation increases to the steady-state rate.
Which statement about a plot of V0 versus [S] for an enzyme that follows Michaelis-Menten kinetics is FALSE?
At very high [S]. the velocity curve becomes a horizontal line that intersectes the y axis at Km