water and inorganic ions
1/23
Earn XP
Description and Tags
learn starred cards - others are just for y12 context/understanding
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
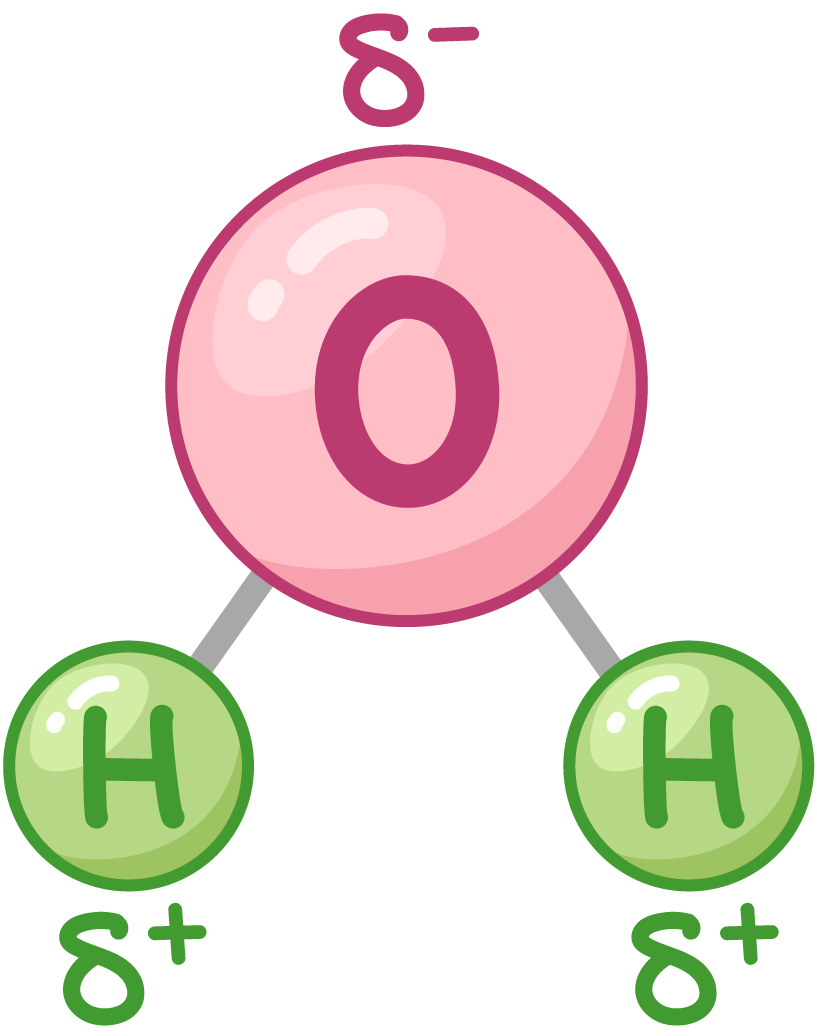
explain fully why water is a dipole:
water molecule is charged - O has a 𝛿- charge and the H atoms have a 𝛿+ charge
what are the key properties of water?
is a metabolite
is a solvent
high specific heat capacity
high latent heat of vaporisation
strong cohesion
why is the e- distribution on a H2O molecule uneven?
O is more electronegative than the H atoms
so -vely charged e- are more attracted to the O atom
explain H bonding between water molecules:
e- are more attracted to the O atom as it is more electronegative
this means that O has a 𝛿- charge and the H atoms have a 𝛿+ charge
the more electronegative 𝛿- O atom becomes attracted to the more electropositive 𝛿+ H atom of another polar molecule
what is the significance of the H bonds between water molecules?
allows for strong cohesion → surface tension
gives water high specific heat capacity - many H bonds can absorb a large amount of energy before being broken
what is cohesion?
the attraction of molecules for other molecules of the same kind
what is the significance of cohesion?
supports columns of water in tube-like transport cells of plants
produces surface tension where water meets air, supporting small organisms
what is adhesion?
the attraction of molecules for other molecules of a different kind
what is surface tension?
where a liquid’s SA is minimised and acts like a skin as a result of cohesion, allowing it to resist an external force (often occurs when water meets air)
give 3 factors in which surface tension is significant:
capillary action
insects walking on water
formation of droplets
how is surface tension significant in capillary action?
can be seen through transpiration: surface tension (and adhesive forces between water and the xylem) allow the transpiration stream to travel up the xylem, against gravity
how is surface tension significant in insects walking on water?
surface tension creates a skin strong enough to support small organism e.g. pond skaters
how is surface tension significant in the formation of droplets?
surface water molecules pull inwards, minimising SA and forming droplets e.g. water beading
what does it mean for water to be a good metabolite?
is used/formed in many metabolic reactions, e.g. condensation and hydrolysis reactions
why is water a good solvent?
ionic compounds and polar molecules can easily dissolve/disassociate in it
this is because water is a polar molecule - +ve end attracts +ve ions and -ve end attracts -ve ions
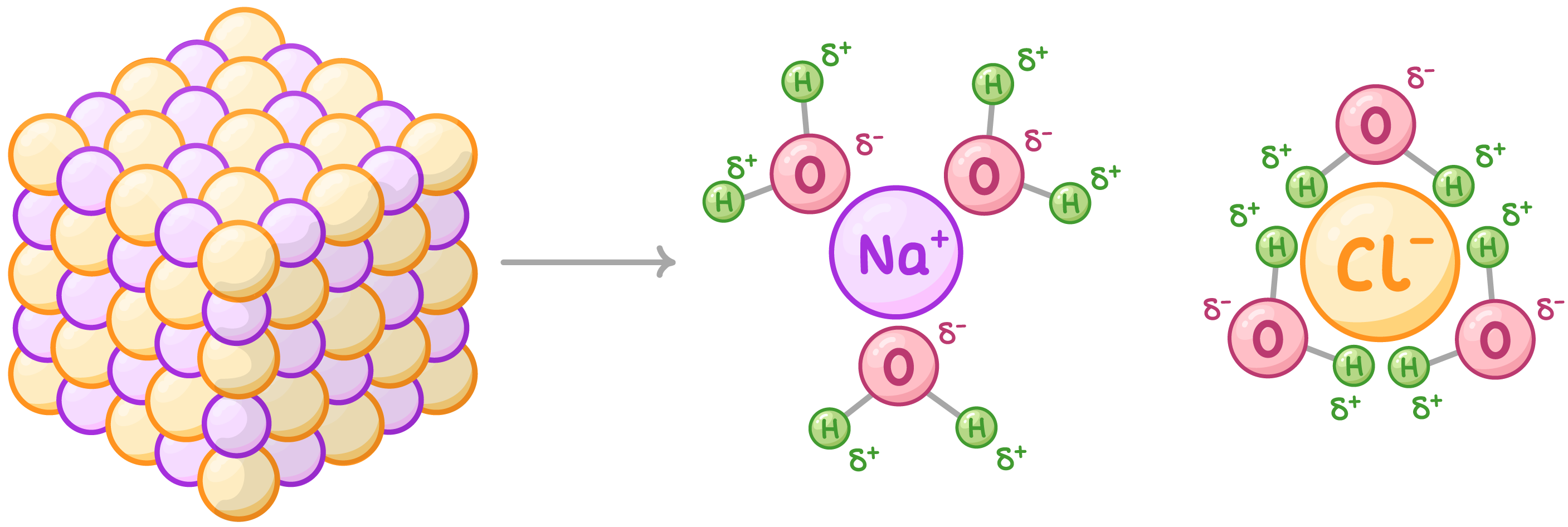
why is it useful for water to be a good solvent?
so metabolic reactions can occur faster in solution
so allowing transport of substances
what does it mean for water to have a relatively high specific heat capacity?
it requires a lot of energy to raise the temperature of 1g of water by 1oC
why is it significant that water has a relatively high specific heat capacity?
buffers changes in temperature (as most organisms consist primarily of water)
takes a lot of heat/energy to change temperature
what does it mean for water to have a relatively large latent heat of vaporisation?
it requires a lot of energy to evaporate 1g of water (high bpt)
why is it significant for water to have a high specific latent heat of vaporisation?
provides a cooling effect (w/ little loss of water) through evaporation
what is the role of Fe2+ ions in a cell?
haemoglobin binds with/associates with/transports/loads oxygen
what is the role of Na+ ions in cells?
co-transport of glucose/AAs (into cells)
Na+ moved out by active transport/Na-K pump
creates a Na+ conc/diffusion gradient
affects osmosis/water potential
what is the role of PO43- ions in cells?
affects osmosis/water potential
joins nucleotides/in phosphodiester bond/in backbone of DNA/RNA/nucleotides
used in/to produce ATP
phosphorylates other compounds making them more reactive
hydrophilic/water soluble part of phospholipid bilayer
compare and contrast the processes by which water and inorganic ions enter cells (3)
