PDA Lecture 32: Biochemical & Metabolic Principles in Toxicology
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
The 3 pathways that some xenobiotics are toxic by:
• Disrupting important metabolic processes,
• Damaging the structure of cells
• Altering the genetic material (DNA) inside cells
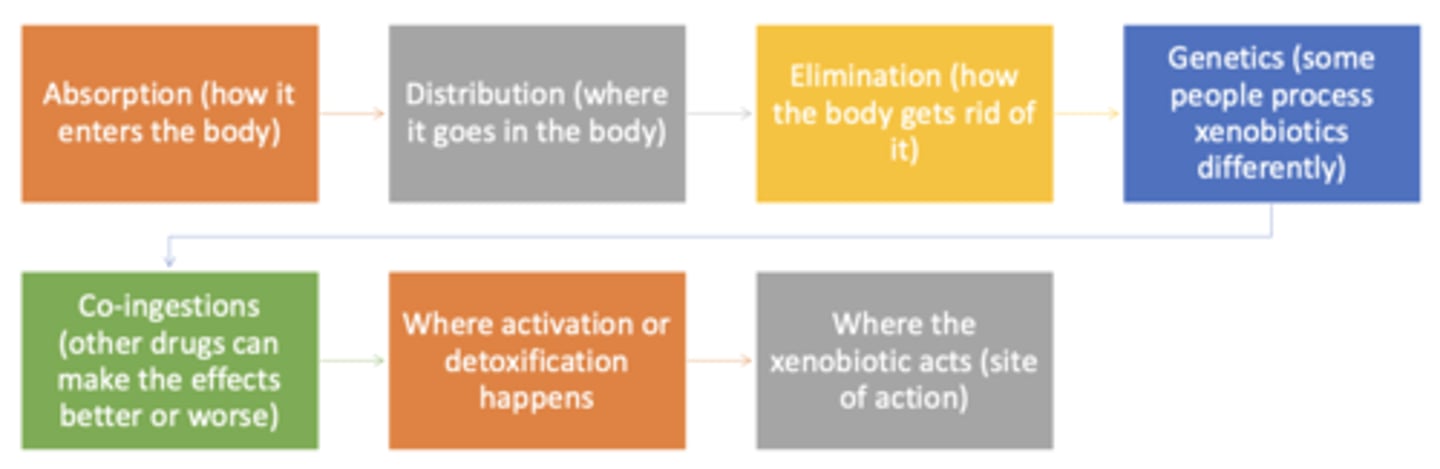
How xenobiotics cause injury (flow chart)
- absorption
- distribution
- elimination
- genetics
- co-ingestions
- where activation or detoxification happens
- where xenobiotic acts
Systemic effects
• Some xenobiotics, once in the bloodstream, affect many organs (cyanide, carbon monoxide).
• The liver is a common target because it receives blood directly from the GI tract and general circulation
How a xenobiotic distributes in the body depends on its: (5)
• Size (molecular weight)
• Protein binding
• Acid/base properties (pKa vs. pH)
• Lipophilicity
• Transport proteins and barriers (like the BBB)
How xenobiotics cause injury: Ionizations and cell entry
Whether a xenobiotic is ionized (charged) or un-ionized (uncharged) matters
Un-ionized xenobiotics are better at...
crossing cell membranes because they are lipophilic
Ionized xenobiotics stay...
stay outside cells
In salicylate poisoning, we alkalinize the urine to...
"trap" the ionized form and help the body eliminate the drug faster
Detoxifying and eliminating toxic substances is essential for...
staying healthy and maintaining homeostasis
What organ has the highest concentration of detoxifying enzymes?
the liver
Inside liver cells (hepatocytes), there are ____________________ depending on the type of chemical
different enzymes
What do Cytosolic enzymes
handle alcohols, aldehydes, esters, and amines
What do Membrane-bound enzymes handle?
lipophilic xenobiotic
Biotransformation
the physiochemical alteration of a xenobiotic, usually as a result of enzyme action
What is the main goal of biotransformation?
to turn lipophilic substances more polar (water-loving) so they can be excreted easily
What does biotransformation reduce?
reduces toxicity by making xenobiotics more hydrophilic (polar) and easier to eliminate
Sometimes, biotransformation can _____________________.
Some xenobiotics are ______________ at first and become toxic only after __________________.
- increase toxicity
- inactive
- metabolism (metabolic activation)
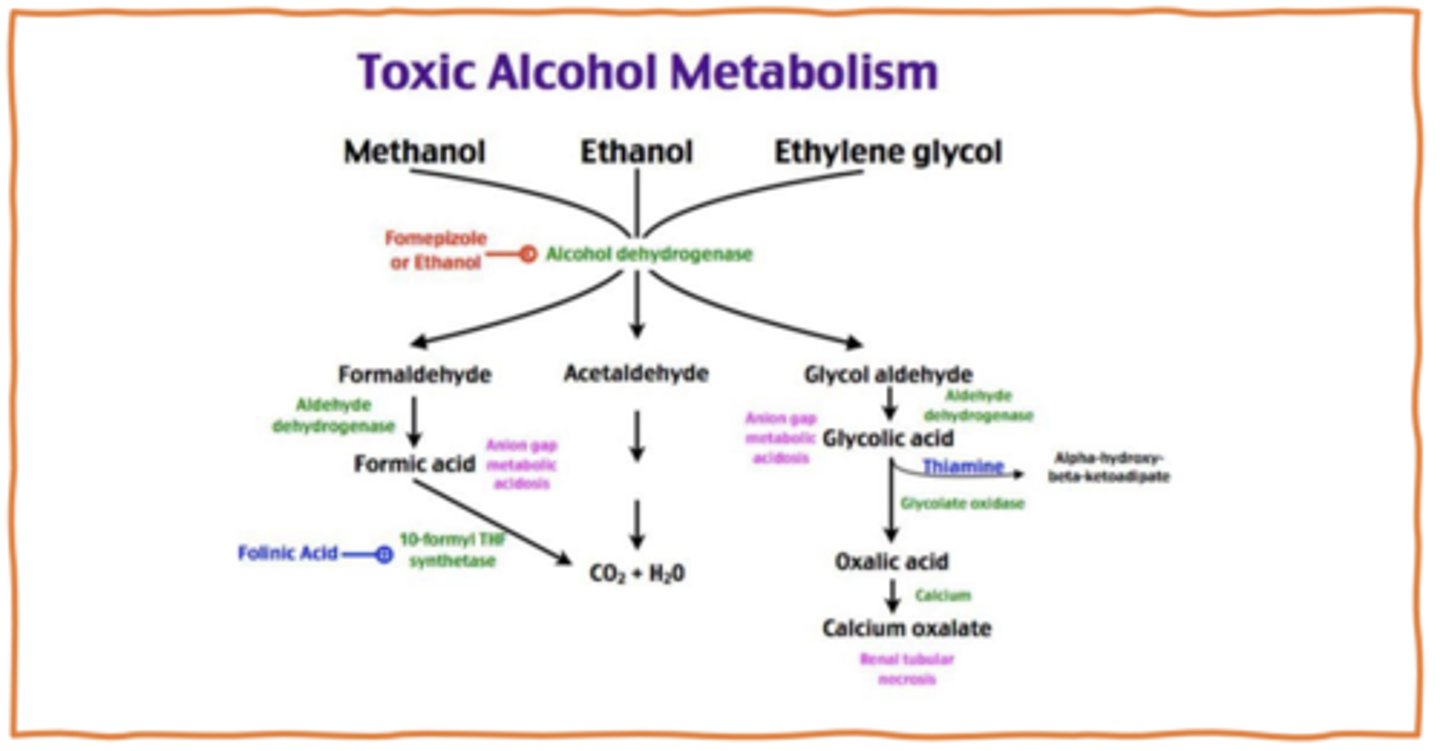
Toxic alcohol metabolism
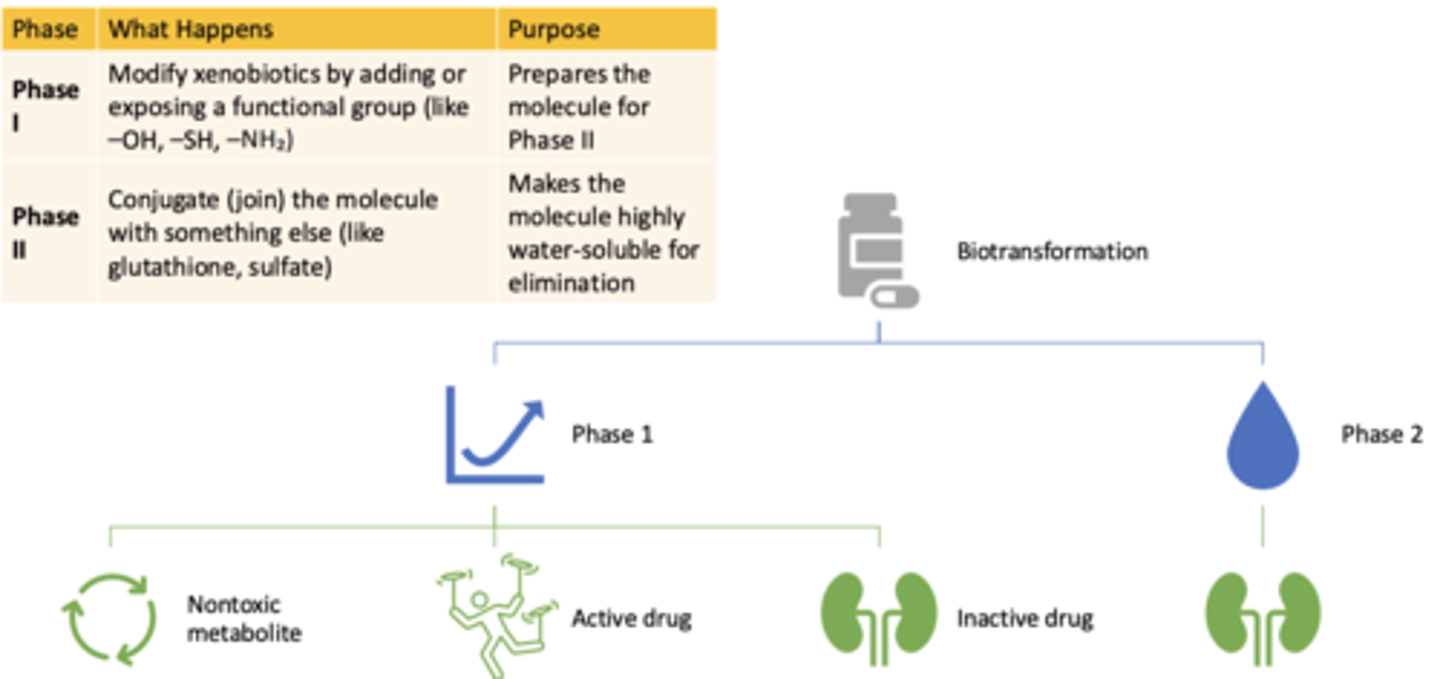
Biotransformation: Phase I
- What happens?
- What is the purpose?
- Modify xenobiotics by adding or exposing a functional group (like -OH, -SH, -NH₂)
- Prepares the molecule for Phase II
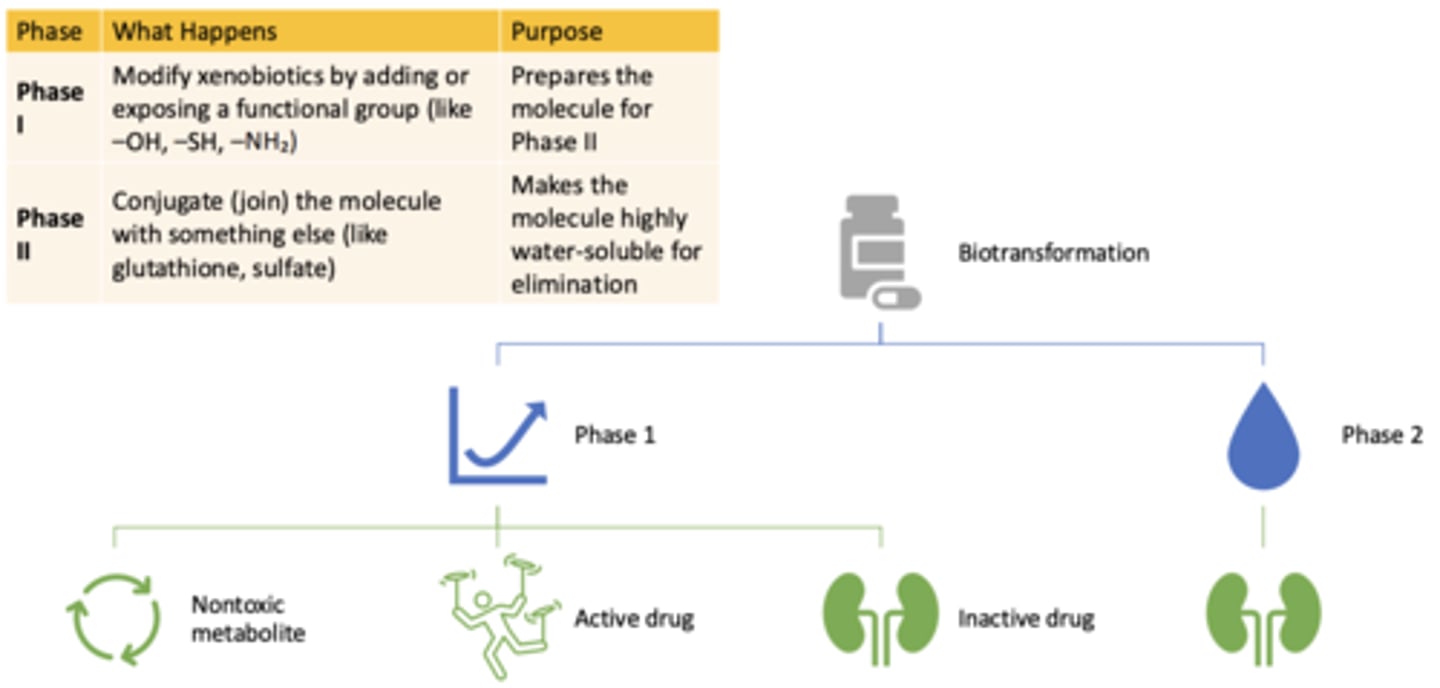
Biotransformation: Phase II
- What happens?
- What is the purpose?
- Conjugate (join) the molecule with something else (like glutathione, sulfate)
- Makes the molecule highly water-soluble for elimination
Drug-drug interactions (DDIs) and adverse drug reactions (ADRs) are common causes of...
hospital visits and harm
- the more meds a pt takes, the higher the risk of these interactions
Up to 50% of ADRs are linked to ____________, especially genes affecting _____________________
- genetics
- drug metabolism
Inhibition vs. Induction:
Inhibitors block ______________ → drug levels ________
Inhibitors block enzyme activity → drug levels go up
Inhibition vs. Induction:
Inducers increase ____________ → drug levels _____________
Inducers increase enzyme activity → drug levels go down
A genetic polymorphism is a DNA variation that occurs in ____% of the population. These can change how well a ____________ works, affecting _____________ and ___________
- ≥1%
- CYP enzyme
- drug metabolism and toxicity
Phenotypes of metabolizers: (4)
- Poor Metabolizers: slow metabolism
- Intermediate: reduced metabolism
- Extensive: normal metabolism
- Ultrarapid: very fast metabolism
A 55-year-old woman with epilepsy is well controlled on carbamazepine. She recently started a new medication, fluoxetine (an antidepressant known to inhibit CYP450 enzymes). Two weeks later, she presents to the emergency department with dizziness, nausea, and confusion. Laboratory studies reveal elevated carbamazepine levels.
Which of the following best explains her clinical presentation?
A) Fluoxetine induced carbamazepine metabolism, leading to toxic accumulation.
B) Fluoxetine inhibited carbamazepine metabolism, leading to toxic accumulation.
C) Fluoxetine induced carbamazepine metabolism, leading to subtherapeutic levels.
D) Fluoxetine had no effect on carbamazepine metabolism
B) Fluoxetine inhibited carbamazepine metabolism, leading to toxic accumulation
A 30-year-old man is prescribed codeine for postoperative pain management. After taking the prescribed dose, he experiences severe drowsiness and signs of opioid toxicity. Genetic testing later reveals that he is an ultrarapid metabolizer of CYP2D6.
Which of the following best explains his symptoms?
A) He metabolized codeine too slowly, resulting in insufficient pain relief.
B) He metabolized codeine too quickly, leading to excessive production of the active drug.
C) He had normal metabolism of codeine, and his symptoms are unrelated.
D) He had poor metabolism of codeine, causing drug accumulation
B) He metabolized codeine too quickly, leading to excessive production of the active drug
Phase I reactions can produce ____________. Ideally, Phase II reactions ________________ these metabolites
- toxic metabolites
- detoxify
T/F: Detoxification always happens
FALSE
doesnt always happen
- some metabolites can cause cellular injury
Sometimes, xenobiotics mimic...
natural substances
Enzymes accidentally transform xenobiotics that mimic natural substances into...
- What can this lead to?
harmful compounds
- can lead to disruption of vital cell functions
Example of the synthesis of toxins: Fluoroacetate (Rodenticide) (3)
- mistaken for a normal molecule in the citric acid cycle
- gets incorporated and disrupts energy production
- leads to cellular toxicity
Metabolic Activation, A Double-Edged Sword
- Many drugs must be....
- What is the problem?
- What does it usually occur through?
• Many drugs and chemicals must be metabolized to be cleared
• Problem: Some metabolic pathways generate toxic metabolites
• This usually occurs through Phase I enzymes, especially CYPs
CYP enzymes and toxicity
High-risk: (4)
Low-risk: (2)
- High-risk: CYP1A1, CYP1B1, CYP2A6, CYP2E1
- Low-risk: CYP2C9, CYP2D6
Toxic metabolites react at or near their site of formation, such as......
liver, lungs, skin, kidneys, GI tract, and nasal mucosa
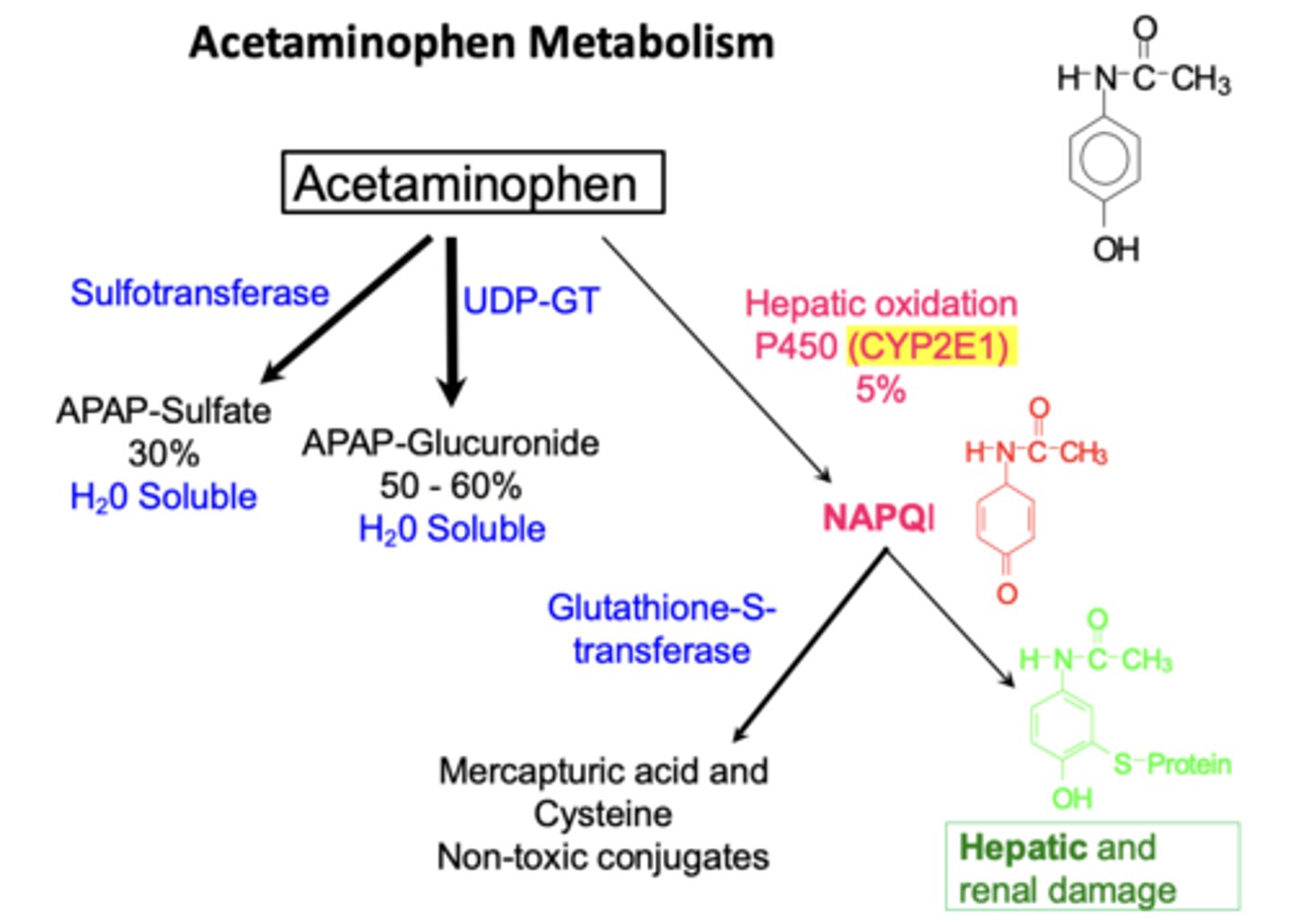
Example: Acetaminophen metabolism (4)
• Normal doses: safely metabolized
• Overdose: excess CYP2E1 generates NAPQI
• NAPQI binds to liver cells causing hepatic injury
• Also formed in kidneys by prostaglandin H synthase causing renal tubular necrosis
What do MAO metabolize?
MAO metabolizes amines: dopamine, serotonin, and some xenobiotics
MAO in glial cells converts __________ into __________
MPTP into MPP+
MPP+ enters ____________________ → inhibits ________________ → causes _______________
- dopaminergic neurons
- mitochondria
- parkinsonism
What are free radicals?
• Atoms/molecules with an unpaired electron
• Highly reactive and unstable
• seek electrons, triggering chain reactions
- ex: superoxide (O₂•−) and hydroxyl radical (HO•)
Oxidative stress occurs when...
What does it lead to?
• When free radical production > antioxidant defenses
• Leads to damage of proteins, DNA, and membranes
What does oxidative stress target?
Polyunsaturated fatty acids (PUFAs) in cell membranes causing lipid peroxidation
What is the result of oxidative stress?
membrane damage→ inflammation → more injury
Glycolysis
What it normally does:
What happens if its affected by overdose:
Result in the body:
What it normally does:
- Breaks down glucose int pyruvate to produce small amounts of ATP
What happens if its affected by overdose:
- Glucose can't be broken down properly → low energy production
Result in the body:
- Fatigue, weakness, lactic acidosis from buildup of pyruvate/lactate
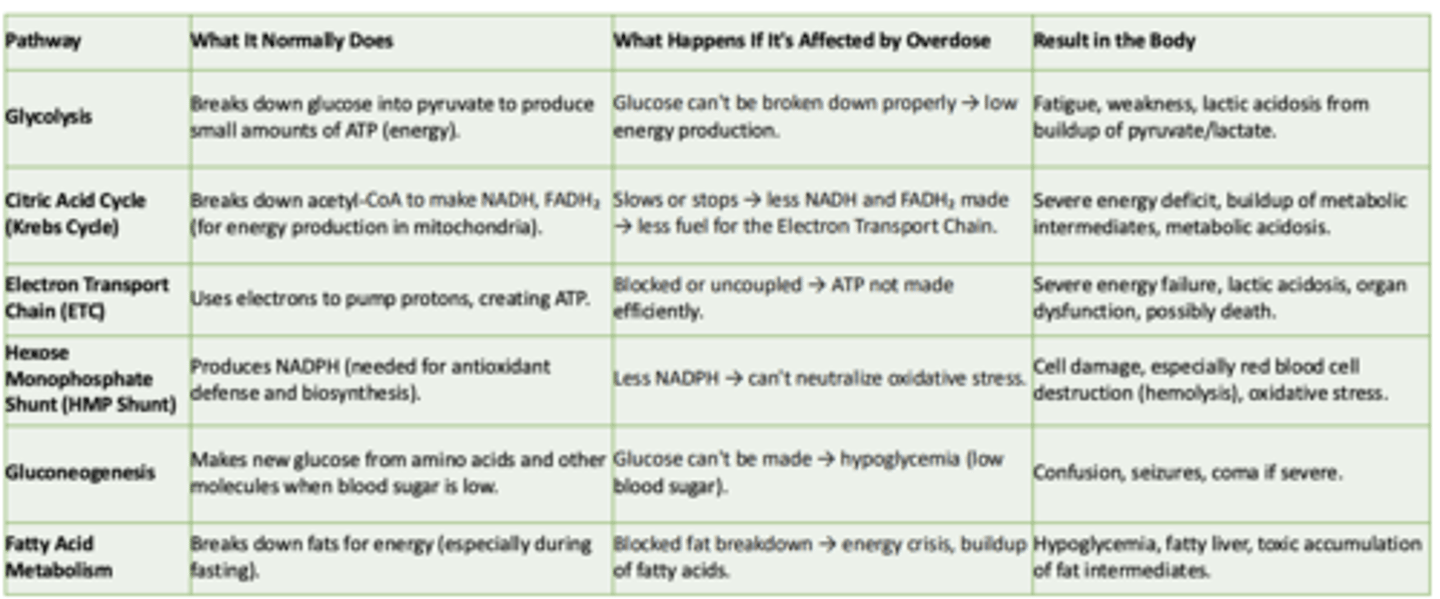
Cirtric Acid Cycle (Krebs Cycle)
What it normally does:
What happens if its affected by overdose:
Result in the body:
What it normally does:
-Breaks down acetyl-CoA to make NADH, FADH₂ (for energy production in mitochondria)
What happens if its affected by overdose:
- Slows or stops → less NADH and FADH₂ made → less fuel for the Electron Transport Chain
Result in the body:
- Severe energy deficit, buildup of metabolic intermediates, metabolic acidosis
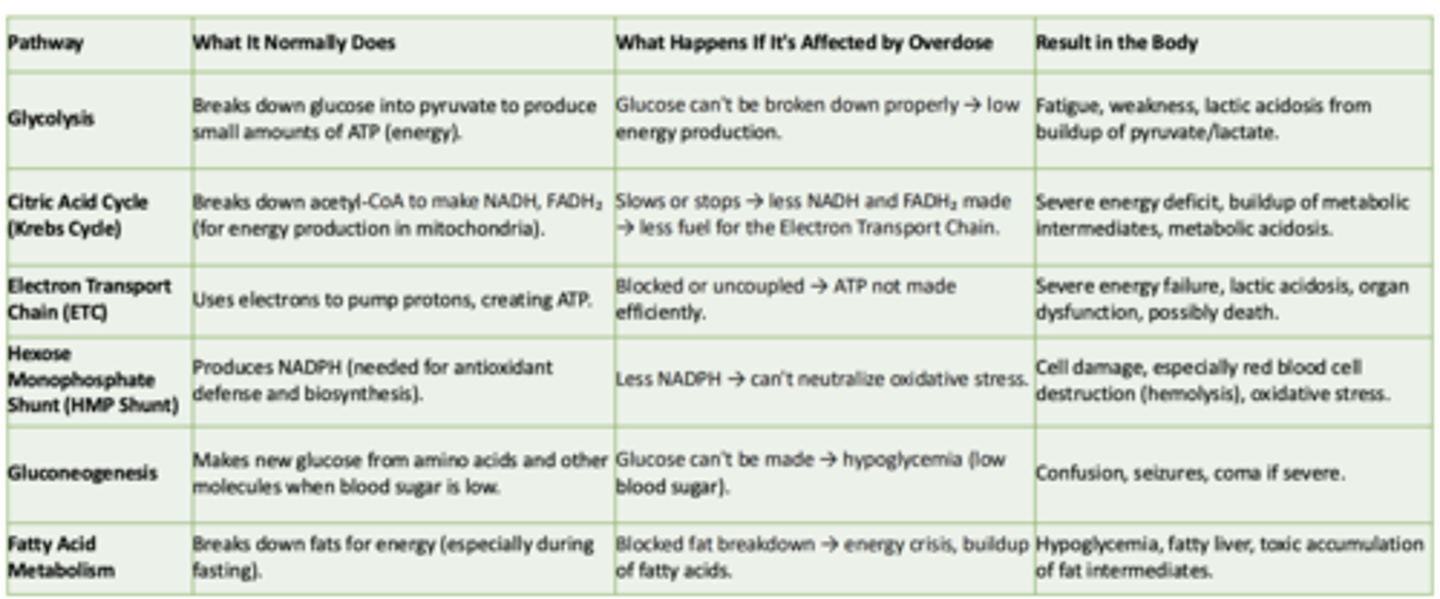
Electron Transport Chain (ETC)
What it normally does:
What happens if its affected by overdose:
Result in the body:
What it normally does:
- Uses electrons to pump protons, creating ATP
What happens if its affected by overdose:
- Blocked or uncoupled → ATP not made efficiently
Result in the body:
- Severe energy failure, lactic acidosis, organ dysfunction, possibly death
Hexose Monophosphate Shunt (HMP Shunt)
What it normally does:
What happens if its affected by overdose:
Result in the body:
What it normally does:
- Produces NADPH (needed for antioxidant defense and biosynthesis)
What happens if its affected by overdose:
- Less NADPH → can't neutralize oxidative stress
Result in the body:
- Cell damage, especially red blood cell destruction (hemolysis), oxidative stress
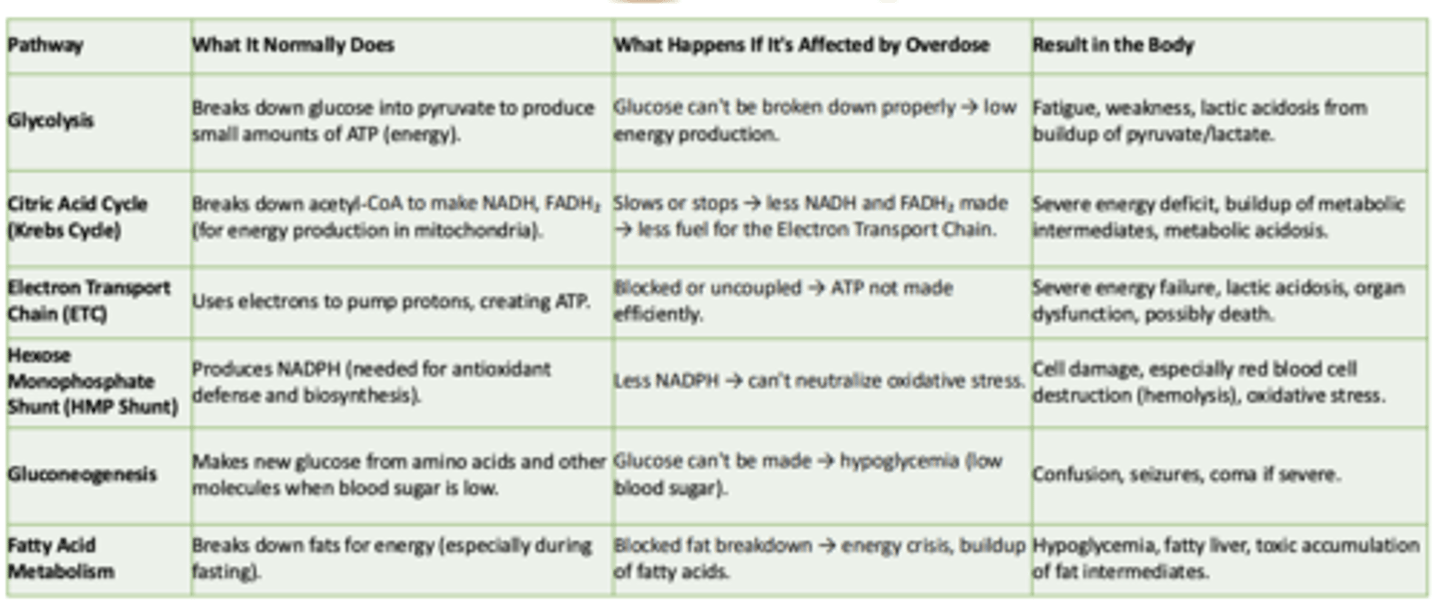
Gluconeogenesis
What it normally does:
What happens if its affected by overdose:
Result in the body:
What it normally does:
- Makes new glucose from amino acids and other molecules when blood sugar is low
What happens if its affected by overdose:
- Glucose can't be made → hypoglycemia (low blood sugar)
Result in the body:
- Confusion, seizures, coma if severe
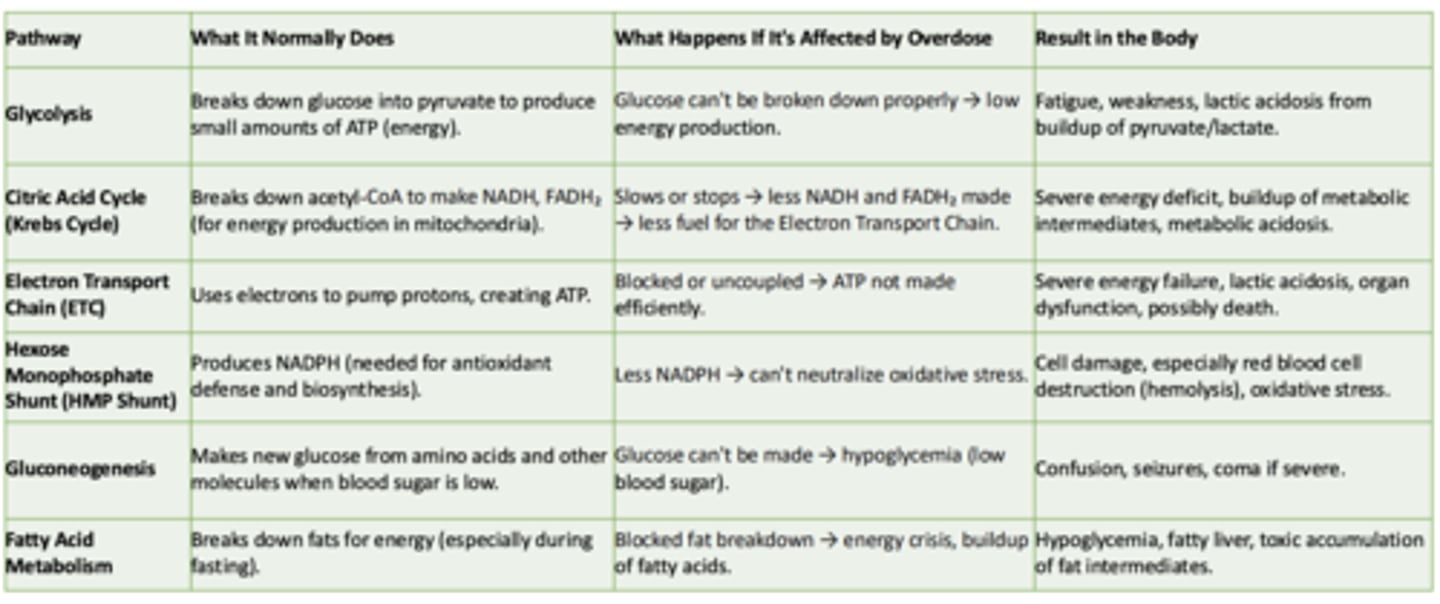
Fatty Acid Metabolism
What it normally does:
What happens if its affected by overdose:
Result in the body:
What it normally does:
- Breaks down fats for energy (especially during fasting)
What happens if its affected by overdose:
- Blocked fat breakdown → energy crisis, buildup of fatty acids
Result in the body:
- Hypoglycemia, fatty liver, toxic accumulation of fat intermediates

Inhibition of the electron transport chain during an overdose can directly lead to:
A. Hyperglycemia
B. Increased ATP production
C. Lactic acidosis and energy failure
D. Enhanced fatty acid breakdown
C. Lactic acidosis and energy failure
How does biotransformation impact drug-drug interactions in toxicology?
A. It causes increased toxicity of all interacting drugs
B. It generally results in decreased toxicity of all interacting drugs
C. It can lead to increased or decreased metabolism of certain drugs, impacting their toxicity or efficacy
D. It has no significant effect on drug interactions
C. It can lead to increased or decreased metabolism of certain drugs, impacting their toxicity or efficacy
What is the potential outcome of inhibition of cytochrome P450 enzymes on drug metabolism?
A. It accelerates the breakdown of drugs, reducing toxicity risks
B. It can lead to increased levels of a drug in the blood, potentially causing toxicity
C. It results in decreased drug bioavailability, reducing its effectiveness
D. It causes enhanced excretion of drugs, leading to reduced toxicity
B. It can lead to increased levels of a drug in the blood, potentially causing toxicity
Which of these substances is most likely to undergo Phase II biotransformation?
A. A lipid-soluble drug requiring conjugation for excretion
B. A water-soluble drug requiring oxidation
C. A drug that requires hydrolysis for activation
D. A drug that needs reduction for proper metabolism
A. A lipid-soluble drug requiring conjugation for excretion