organic chemistry 4: analyzing organic reactions
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
[...] acids are electron acceptors
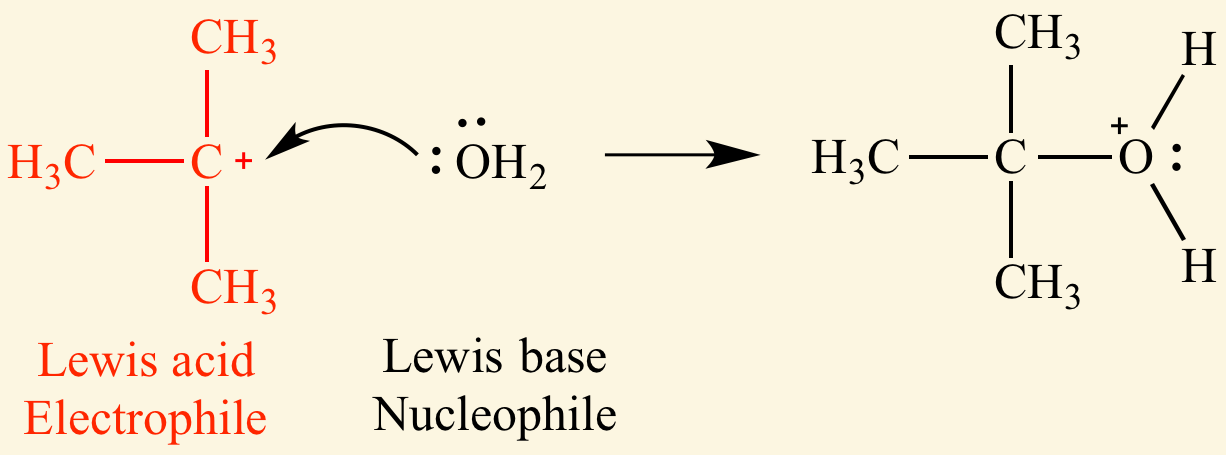
lewis acids
contains vacant orbitals or postive polarized atoms
lewis acids are electron acceptors
[...] bases are electron donors
lewis bases
contain a lone pair of electrons and are often anions
A/an [...] acid is a proton donor
Bronsted-Lowry acid
A/an [...] base is a proton acceptor
A/an Brønsted-Lowry base is a proton acceptor
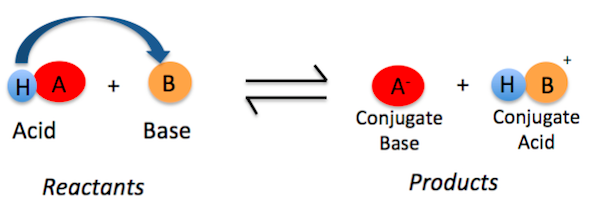
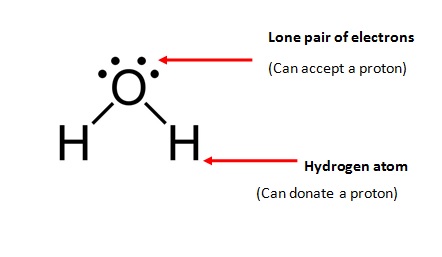
[...] molecules can act as either acids or bases
amphoteric
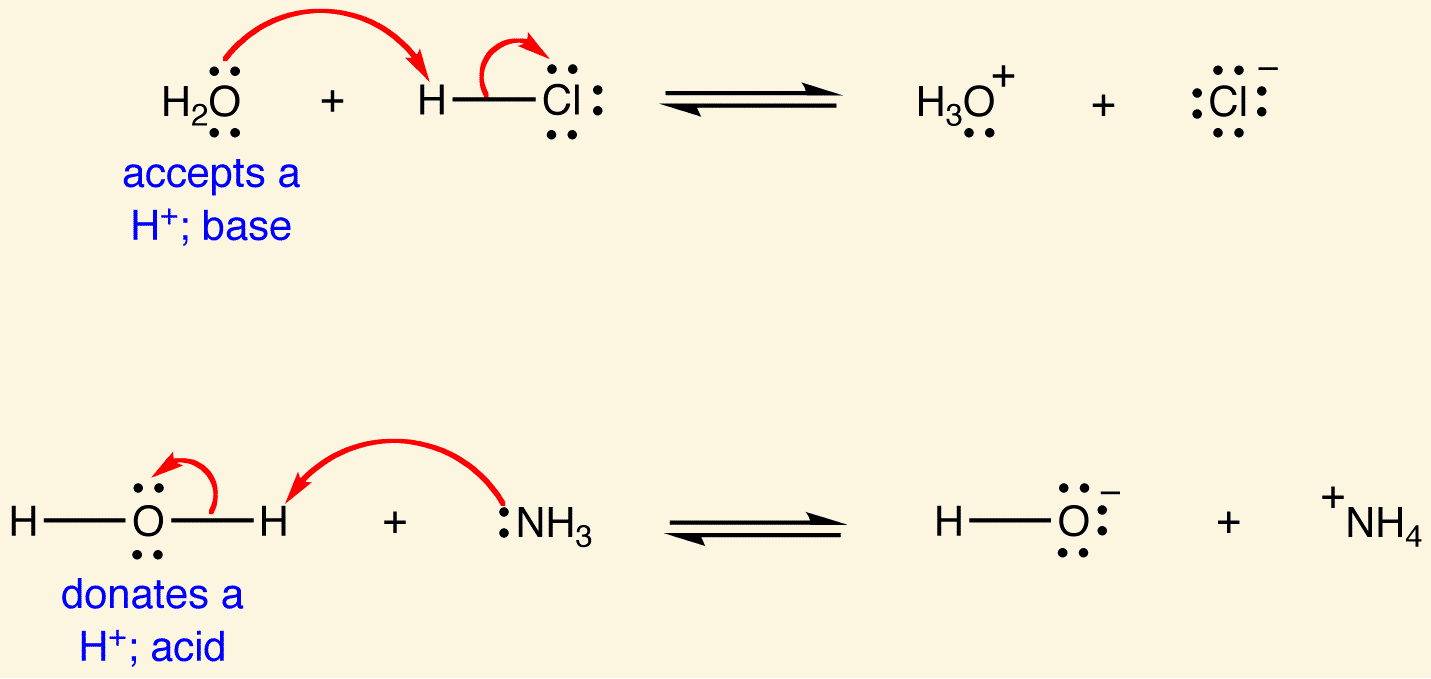
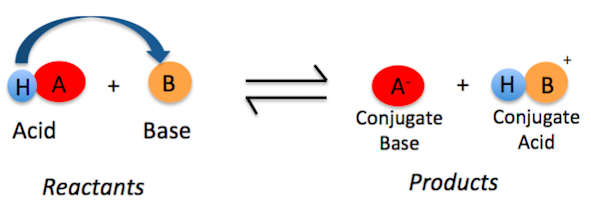
Ka is the [...] constant
acid dissociation
an indicator of acid strength
it is the equilibrium constant corresponding to the dissociation of an acid, HA into a proton and its conjugate base
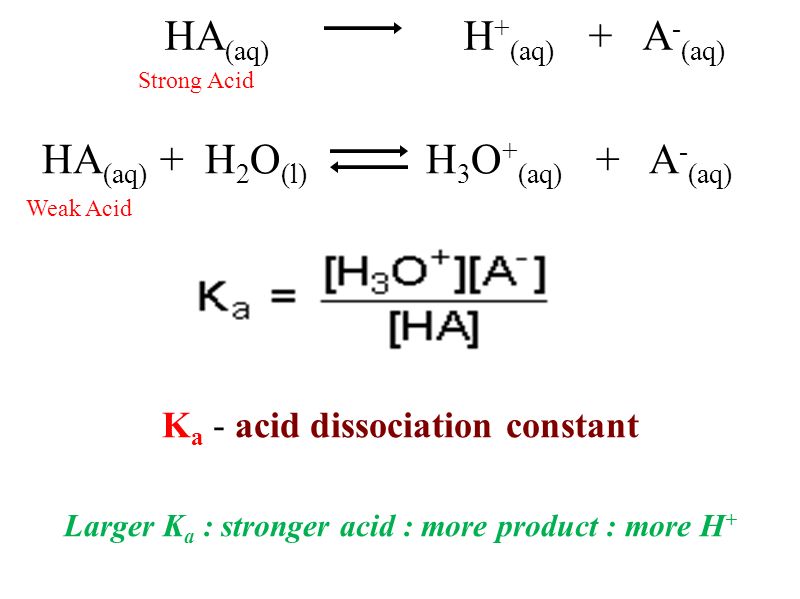
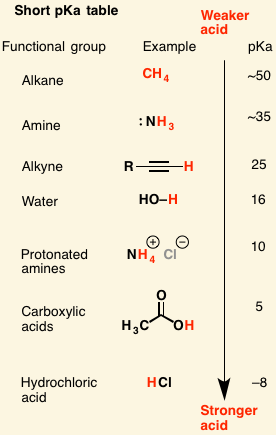
Low pKa = [weak or strong] acid
strong
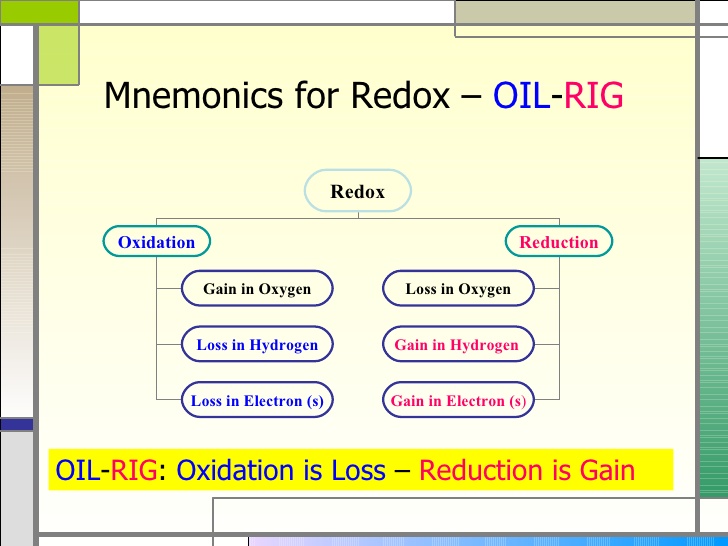
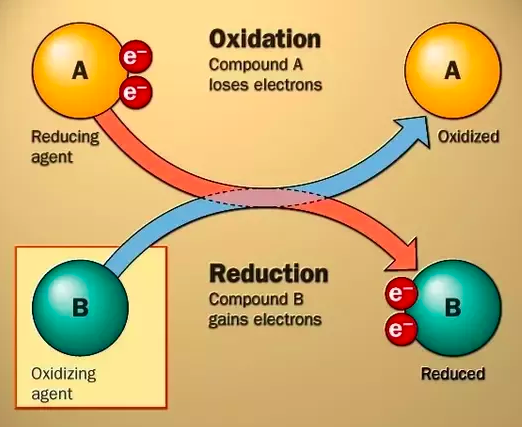
A/an [...] reaction is a reaction with the transfer of electrons from the substance being oxidized to the substance being reduced
redox
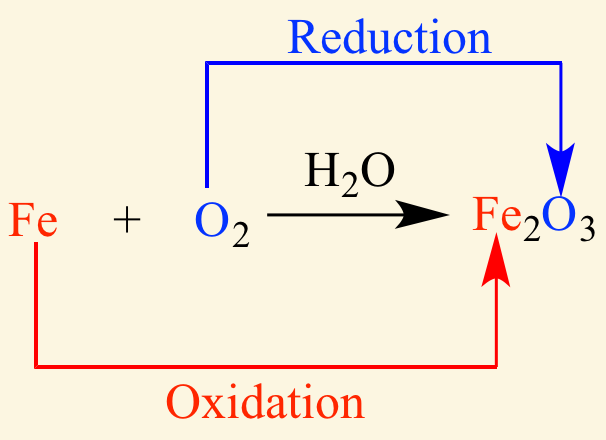
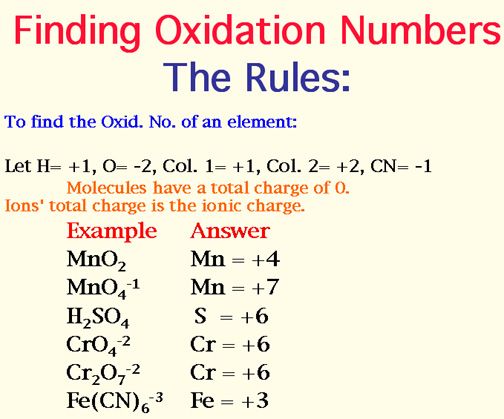
[...] is the charge an atom would have if all its bonds were completely ionic
oxidation number
In REDOX reactions, the [oxidized or reduced] molecule loses electrons and the oxidation number increases
oxidized
An oxidizing agent [accepts or donates] electrons and is [oxidized or reduced] in the process
accepts; reduced
n REDOX reactions, the [oxidized or reduced] molecule gains electrons and the oxidation number decreases
reduced
A reducing agent [accepts or donates] electrons and is [oxidized or reduced] in the process
donates
oxidized
[...] is the ability of a reagent or intermediate to react with one group or atom in a molecule in preference to another group or atom present in the same molecule
chemoselectivity
A/an [...] contains lone pairs of electrons or pi bonds and is “Nucleus-loving”
nucleophile
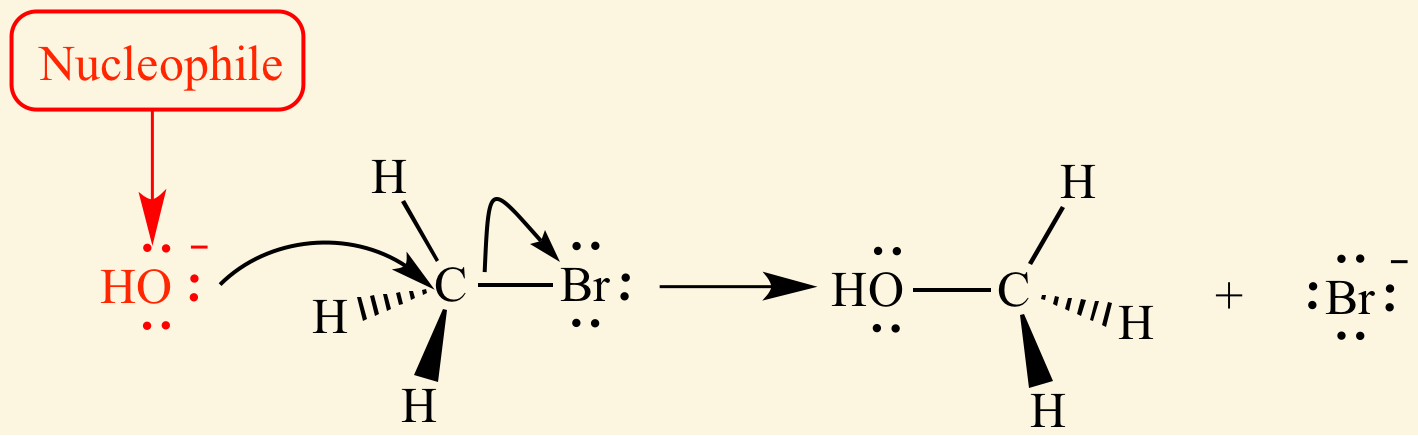
[...] is a term used to describe the strength of a nucleophile
nucleophilicity
nucleophilicity is affected by:
charge
electronegativity
steric hindrance
solvent
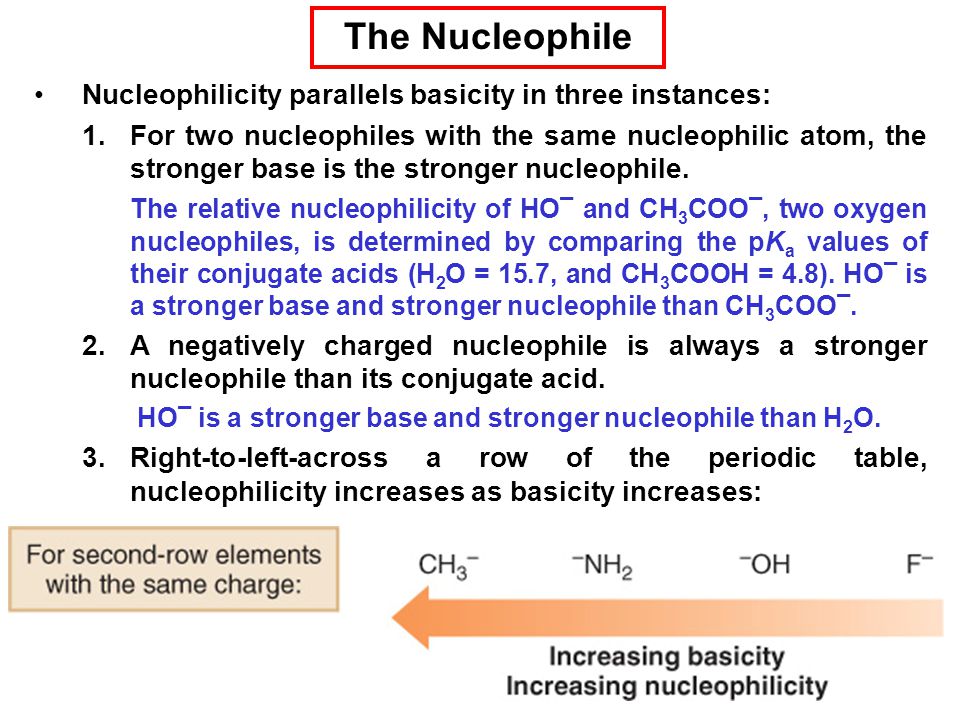
[...] are electron loving
electrophiles
contain a positive charge or are positively polarized
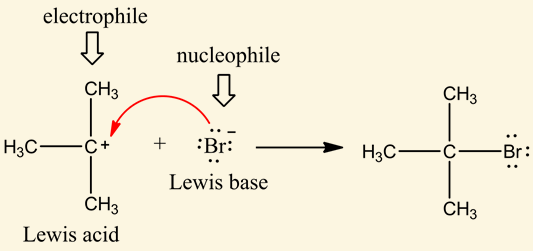
[...] are molecular fragments that retain the electrons after heterolysis
leaving groups
the least LG can stabilize additional charge through resonance of induction
weak bases make good leaving groups
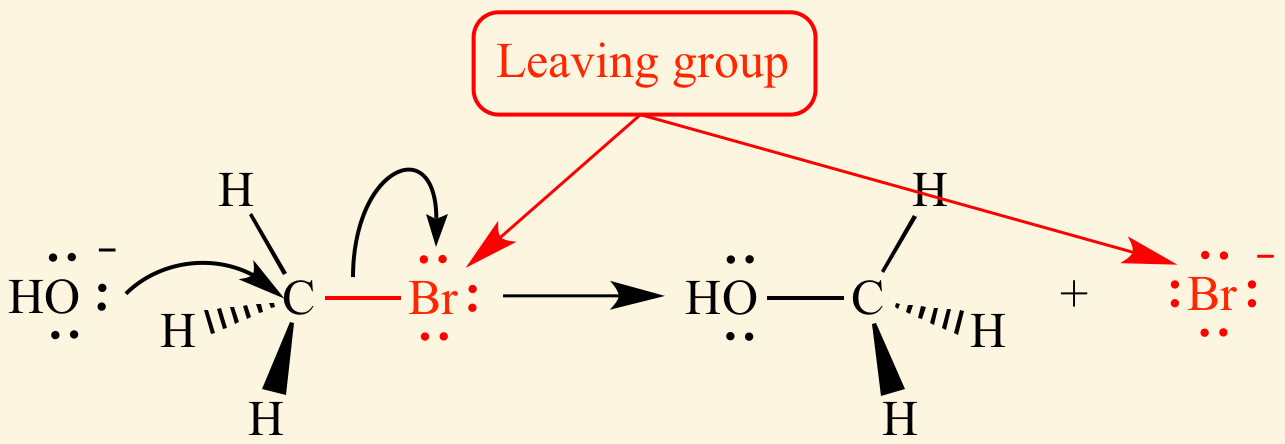
Cl- and Br- are [good or bad] leaving groups
good
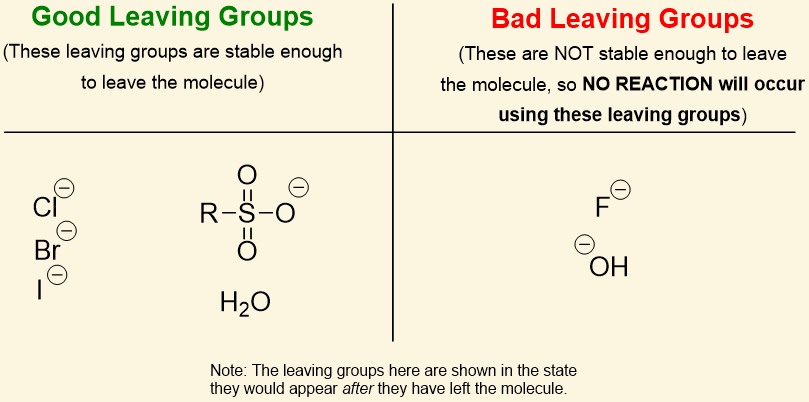
-OH is a[good or bad]leaving group
bad
The "SN" in SN1 and SN2 reactions refers to [...]
nucleophilic substitution
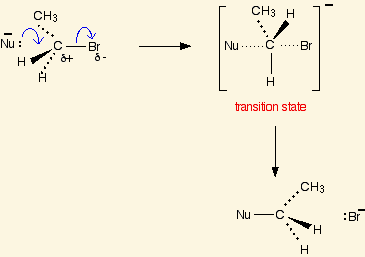
The number 1 in an SN1 reaction refers to the fact that the reaction is [...]
unimolecular
SN1 rate = k(substrate)1 = a first-order reaction
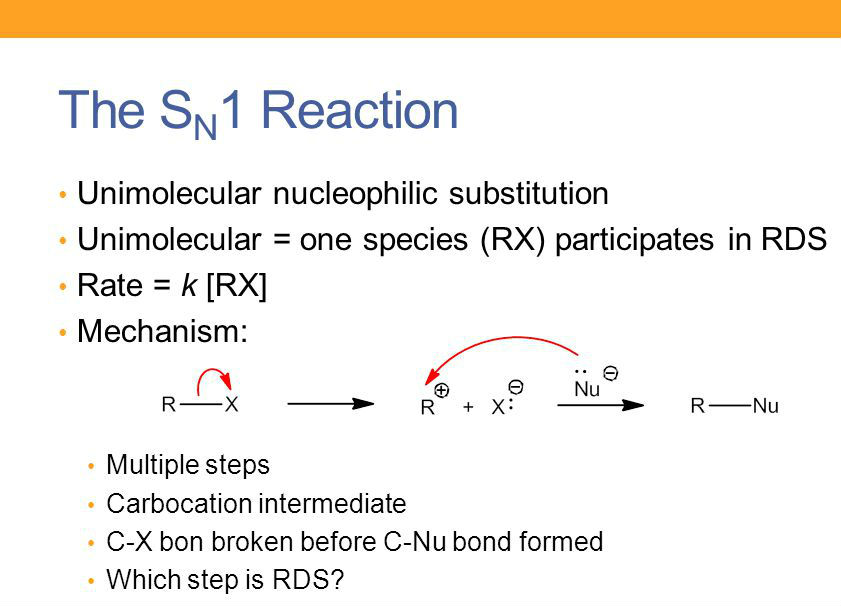
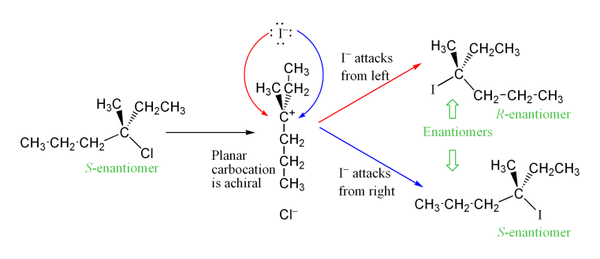
An SN1 reaction has [#] step(s)
two
step 1: the LG leaves, forming a carbocation
step 2: the nucleophile attacks the planar carbocation from either side, leading to a racemic mixture of products
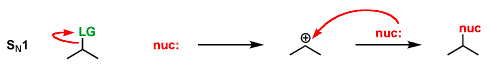
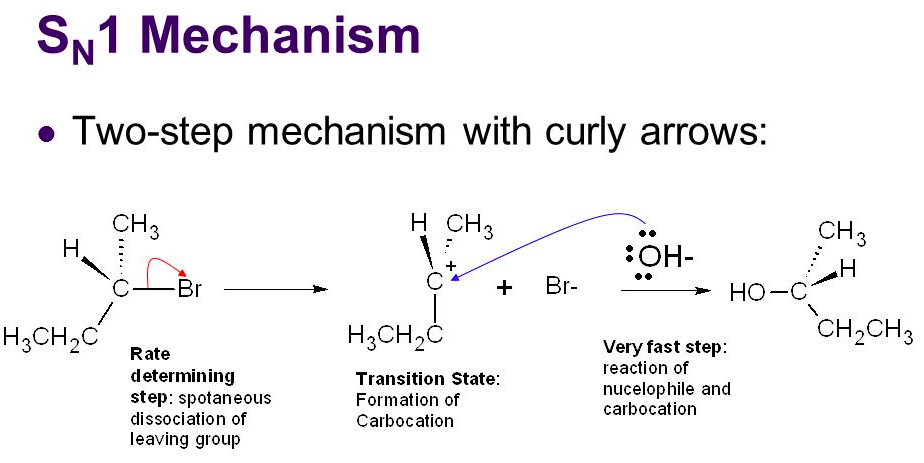
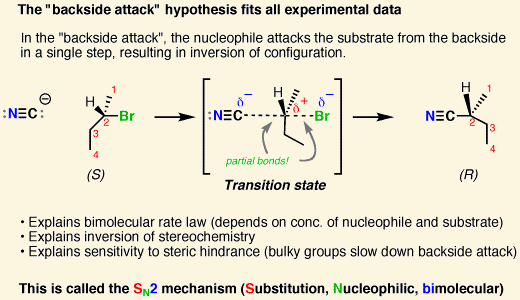
An SN2 reaction has [#] step(s)
one
the nucleophile attacks at the same time as the lG leaves
the nucleophile must perform a backside attack, which leads to inversion of stereochemistry
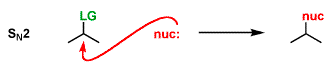
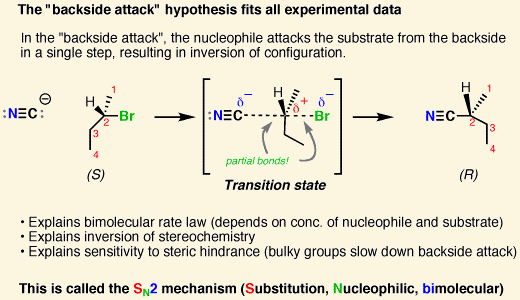
[SN1 or SN2] products are a racemic mixture
SN1
in the 2nd step, the nucleophile attacks the planar carbocation from either side, leading to a racemic mixture of products
[SN1 or SN2] reactions lead to inversion of stereochemistry
SN2
the nucleophile must perform a backside attack, which leads to inversion of stereochemistry
The rate law for an SN1 reaction is [...]
SN1 Rate = k[substrate]1
A first-order reaction
![<p><span style="color: mediumseagreen"><strong>S<sub>N</sub>1 Rate = k[substrate]<sup>1</sup></strong></span></p><p> </p><p>A <strong>first-order</strong> reaction</p>](https://knowt-user-attachments.s3.amazonaws.com/0f3798d7-b2f9-4a3a-9bad-088626c7fef6.jpg)
The rate law for an SN2 reaction is [...]
SN2 Rate = k[substrate]1[nucleophile]1
A second-order reaction
![<p><span style="color: mediumseagreen"><strong>S<sub>N</sub>2 Rate = k[substrate]<sup>1</sup>[nucleophile]<sup>1</sup></strong></span></p><p> </p><p>A <strong>second-order</strong> reaction</p>](https://knowt-user-attachments.s3.amazonaws.com/c727a61f-403d-4463-82f1-03619752fa08.jpg)
![<p><span>A </span><strong><u>primary</u></strong><span> substrate will go </span><span style="color: mediumseagreen"><strong>[S<sub>N</sub>1 or S<sub>N</sub>2]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/982595da-07f4-419f-beb4-36d12a454f71.png)
A primary substrate will go [SN1 or SN2]
Sn2
1 step: if you were do an do an sn1 reaction on a primary substrate you’d get a primary carbocation which is very unstable

![<p><span>A </span><strong>tertiary</strong><span> substrate with a protic (or aprotic) solvent will go </span><span style="color: mediumseagreen"><strong>[S<sub>N</sub>1 or S<sub>N</sub>2]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/056b1af3-1090-4323-8bb7-215ac72dff61.png)
A tertiary substrate with a protic (or aprotic) solvent will go [SN1 or SN2]
sn1
2 steps
there is to much steric hinderance for the nucleophile to attack the carbon while the leaving group is still there. The LG must leave first
the products will be a mixture of sn1 and e1

theis reaction will go
sn1
the substrate is secondary to CH3OH is a protic solvent
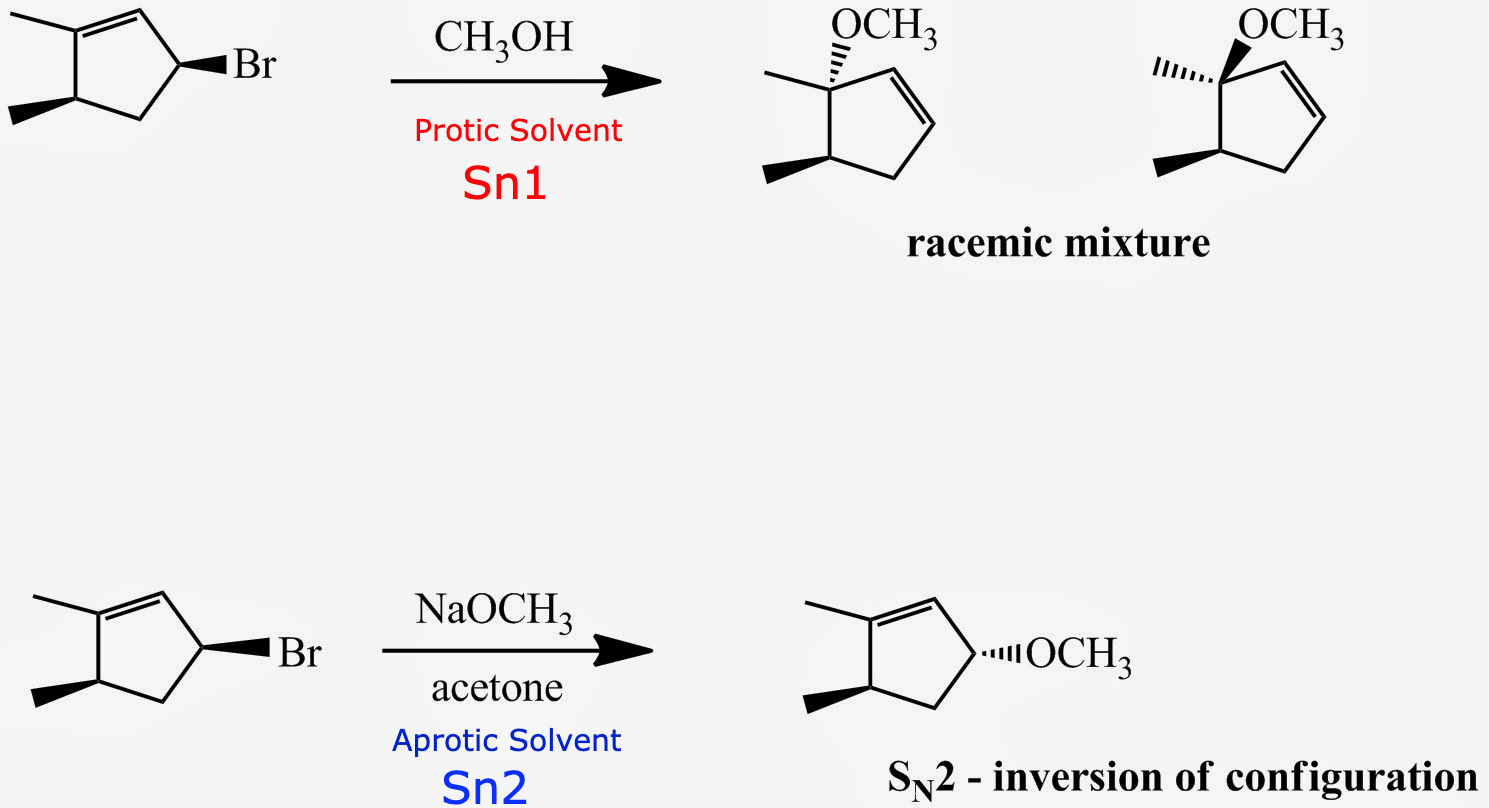
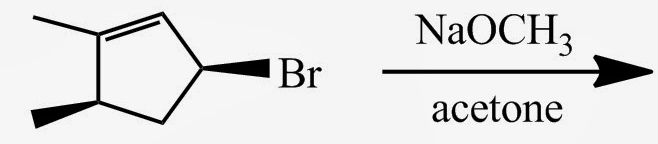
this reaction will go
sn2
the substrate is secondary and acetone is an aprotic solvent

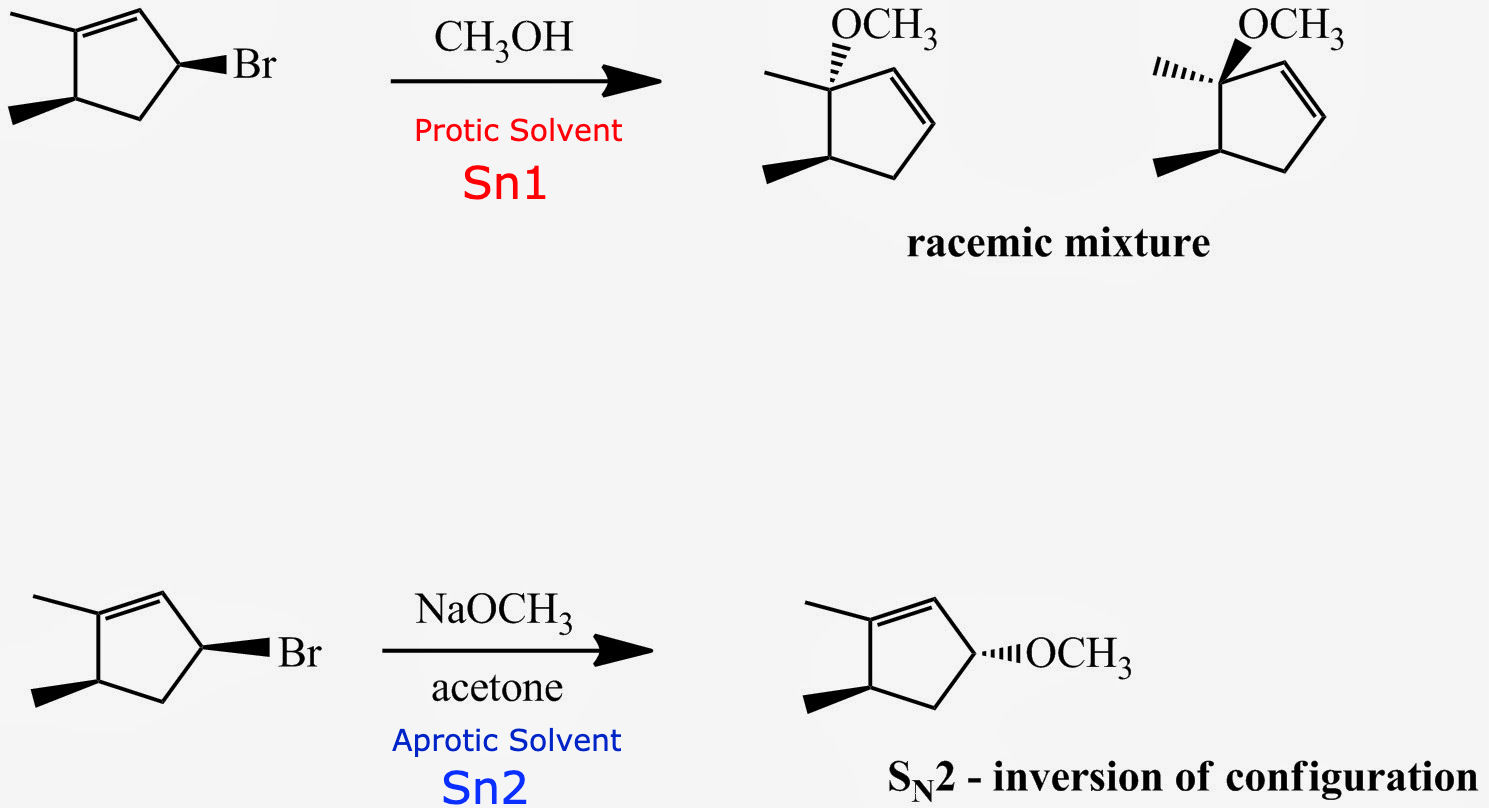
SN2 reactions prefer[...]substrates
methyl and primary
it is easy for the nucleophile to attack the carbon because the carbon is accessible (less steric hindrance)
the reaction can process in 1 step

![<p><span>A </span><strong><u>methyl</u></strong><span> substrate </span><strong>cannot</strong><span> undergo an </span><strong>elimination</strong><span> reaction because elimination reactions create </span><span style="color: mediumseagreen"><strong>[...]</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/072b4ff7-5518-48f5-a613-6d10bbd8fe16.png)
A methyl substrate cannot undergo an elimination reaction because elimination reactions create [...]
double bonds between two carbons, C=C
there is only one carbon in methyl so no double bond can be created

Strong bases favor [...] reactions
E2

A secondary substrate with a protic solvent will go [SN1 or SN2]
Sn1
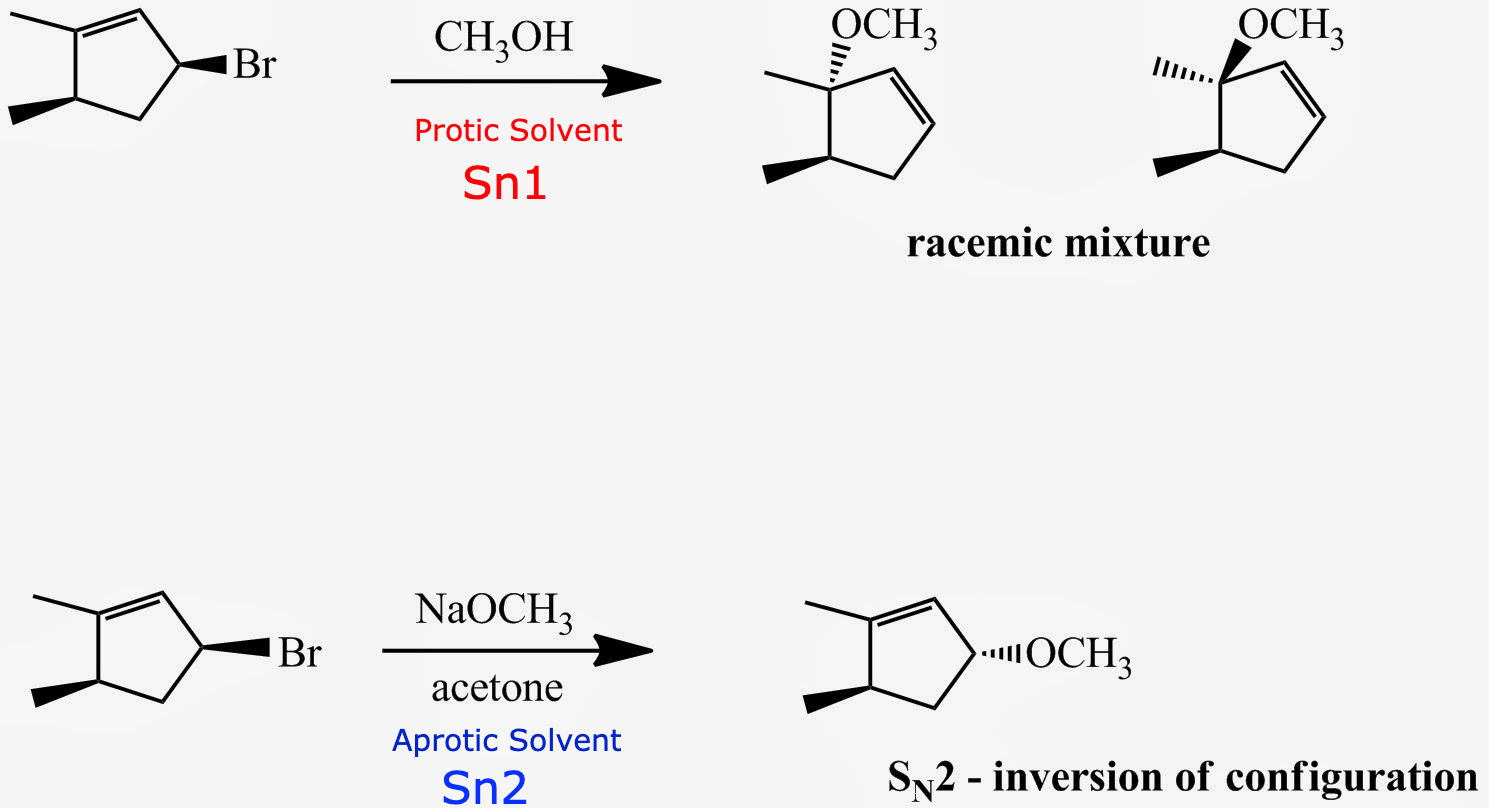
A secondary substrate with an aprotic solvent will go [SN1 or SN2]
sn2

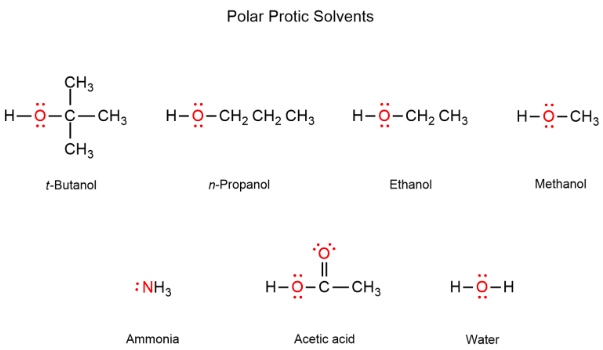
Polar [protic or aprotic] solvents are capable of hydrogen bonding
protic
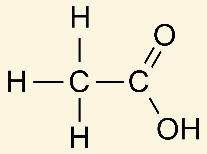
Polar Protic: Acetic Acid, H2O, ROH, NH3
Polar [protic or aprotic] solvents do not participate in hydrogen bonding
aprotic
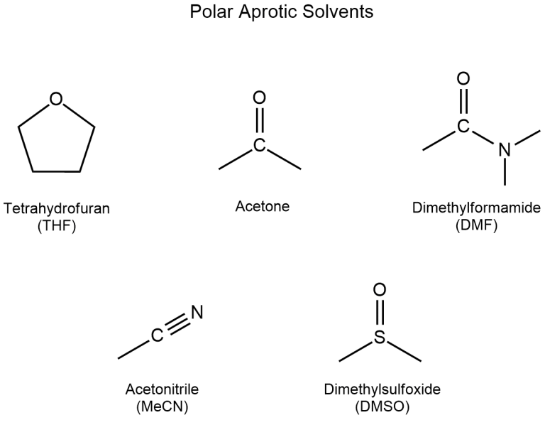
Polar Aprotic: DMF, DMSO, Acetone, Ethyl Acetate
Acetic acid is polar [protic or aprotic]
protic
polar protic because it participates in hydrogen bonding
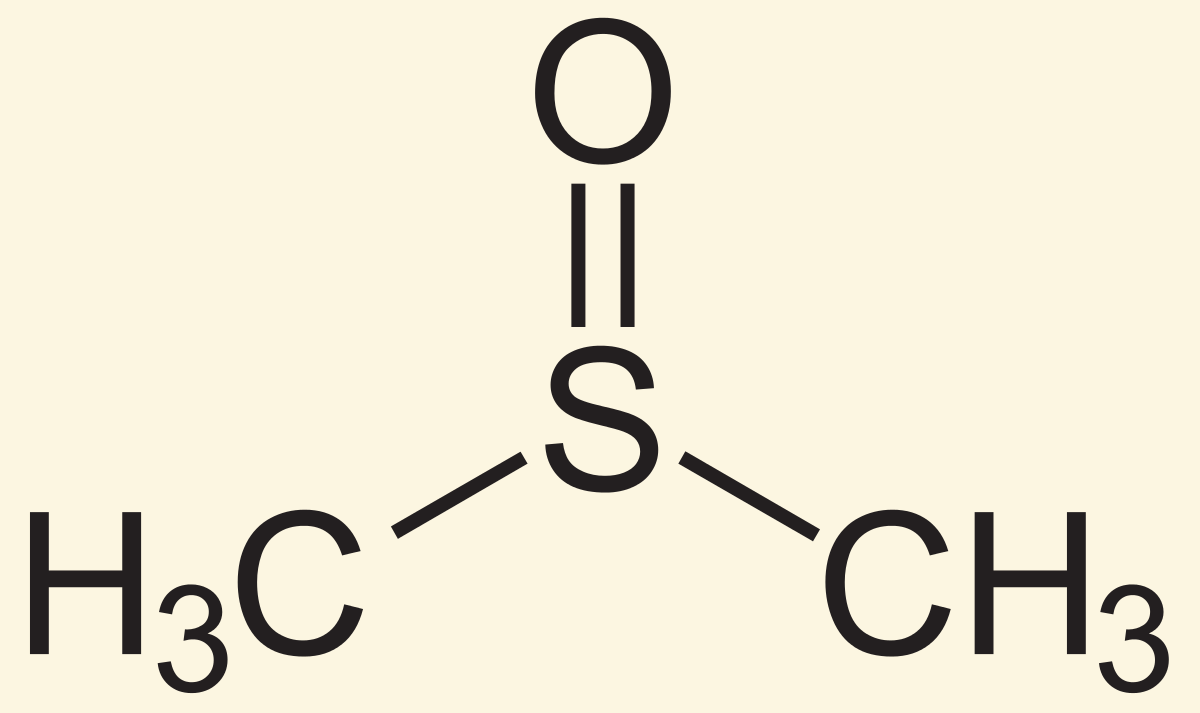
DMSO is polar [protic or aprotic]
aprotic
-dimethyl sulfoxide is polar aprotic because it does not participate in hydrogen bonding