Redox chemistry
An oxidising agent is a substance that causes or promotes the oxidation of a metal to produce a metal compound.
Double displacement reactions do not participate in redox reactions
Redox reactions involve the transfer of electrons from a reducing agent to an oxidising agent.
oxidations lose electrons
Reductions gain electrons
redox reactions can be divided into 2 parts: half-reactions
A half-reaction represents what happens to only one reactant in an overall reaction.
oxidising half
, reducing half
When balancing half-reactions, the total number of electrons gained/removed must be equal to the electrons gained/removed on the other half
the total number of electrons gained in a reaction must equal the total number of electrons lost.
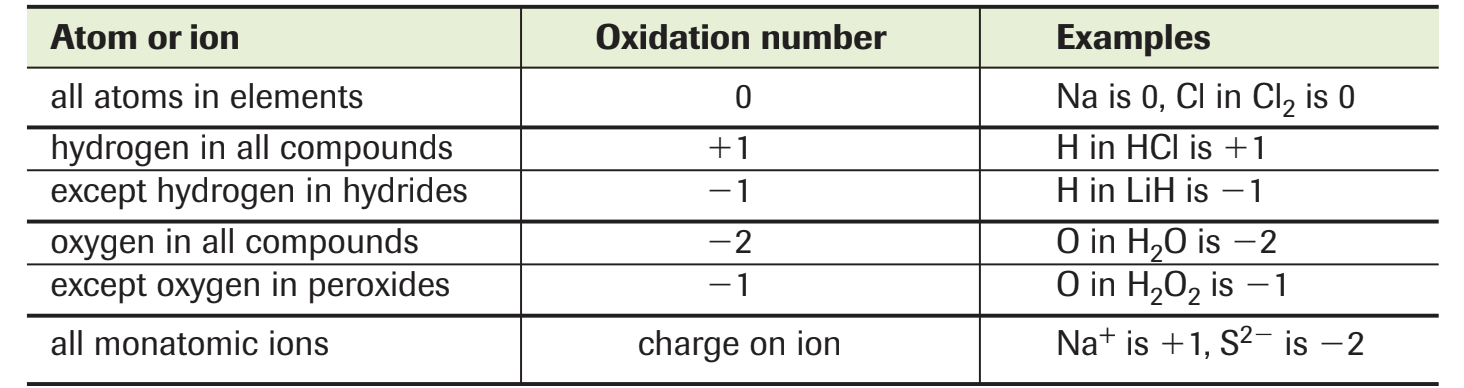
An oxidation is an increase in oxidation number.
A reduction is a decrease in oxidation number.
To balance redox reactions:
Write the chemical formulas for the reactants and products.
Balance all atoms other than O and H.
Balance O by adding H2O(l).
Balance H by adding H(aq).
Balance the charge on each side by adding e and cancel anything that is the same on both sides.
For basic solutions only,
Add OH(aq) to both sides to equal the number of H(aq) present.
Combine H(aq) and OH(aq) on the same side to form H2O(l). Cancel equal amounts of H2O(l) from both sides.
The molecule that is being reduced (or undergoing reduction) is the oxidising agent.
The molecule that is being oxidised (or undergoing oxidation) is the reducing agent