AP Chem Molecular Geometry
1/14
Earn XP
Description and Tags
Flashcards about VSEPR Theory
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Lone Pairs
Atoms that are not bonded to other atoms.
Molecular Geometry
Touching atoms in a molecule determine its geometry.
Angle
The angle between bonded atoms.
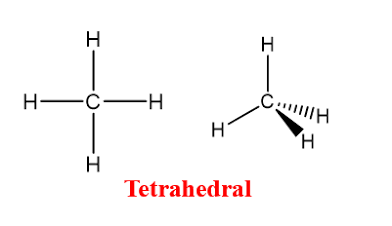
Tetrahedral
Four bonded atoms, no lone pairs, and a bond angle of 109.5°, sp3
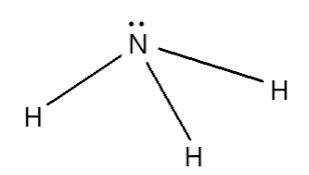
Trigonal Pyramidal
three bonded atoms and one lone pair, bond angle of approximately 109.5°, sp3
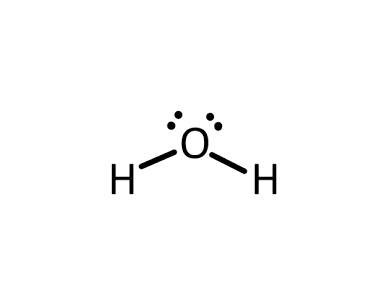
Bent
two bonded atoms and two lone pairs, bond angle of approximately 109.5°, sp3
Trigonal Planar
three bonded atoms, zero lone pairs, bond angle of 120°, sp2
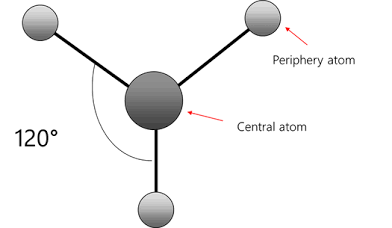
Bent / Angular
Molecular geometry with two bonded atoms and one lone pair, resulting in a bond angle of approximately 120°.
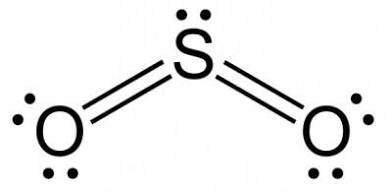
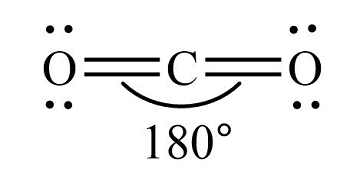
Linear
Two bonded atoms, no lone pairs, and a bond angle of 180°, sp
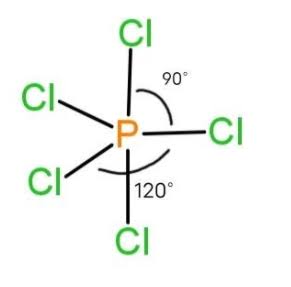
Trigonal Bipyramidal
Five bonded atoms, no lone pairs, and bond angles are 90° & 120°, sp3d
See-saw
Four bonded atoms, one lone pair, and bond angles are ~90° & 120°, sp3d
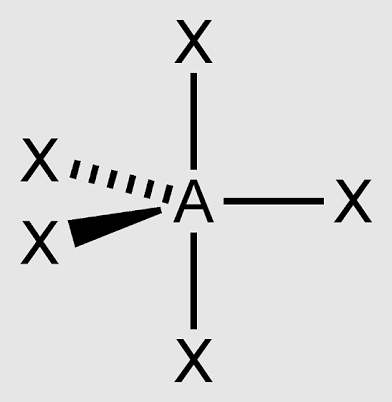
T-shaped
3 bonded atoms, two lone pairs, and bond angles of 90°, sp3d
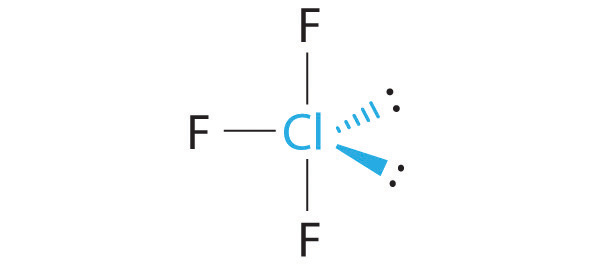
Octahedral
6 bonded atoms, no lone pairs and bond angles of 90°, sp3d2
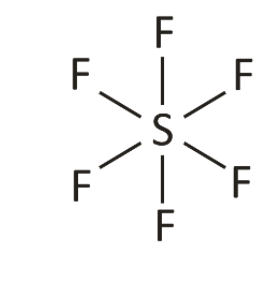
Square Pyramidal
5 bonds, one lone pair, bond angles of ~90°, sp3d2
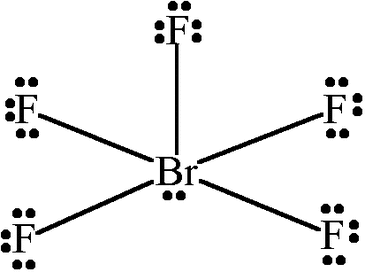
Square Planar
4 bonded atoms, two lone pairs, and bond angles of 90°, sp3d2