MD1: SAR of B-agonists and antimuscarinics
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
PNS drug targets
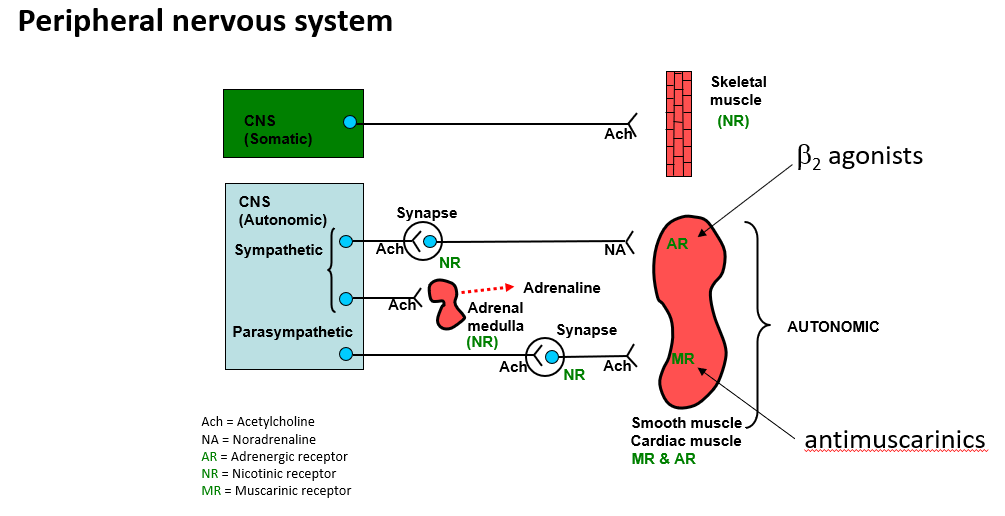
Examples of respiratory drugs
Salbutamol mimetic of A/NA binds to B2 receptor
Ipratropium binds to muscarinic receptor
•Short onset, short duration of action bronchodilators.
•Treatment of symptoms of asthma and chronic obstructive
pulmonary disease (COPD).
•Provide short-term relief.
Explain the 3 stages in SAR
•Start from a lead compound, establish SAR.
•Make specific structural modifications to the lead to convert it into a selective agonist or antagonist, such as…
• Chain extension, conformational restriction, group shifting, chiral switching.
Lead compounds
Prototype chemical structure with desirable biological activity
Found in :
Natural receptor ligands, ACH, NA
Natural products e.g. muscarine
Describe the chemistry functional groups in Ach
Non covalent interactions:
Ester: H-bond interaction
N+ ionic interaction
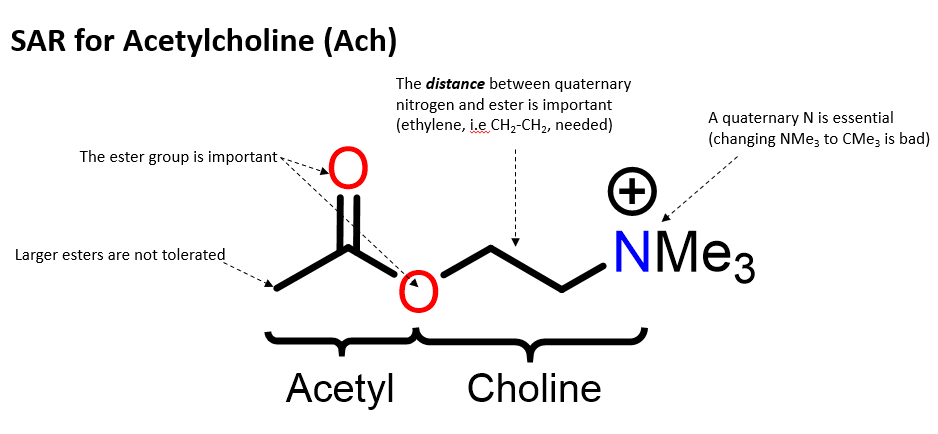
Describe function of CH3, Ester, Nitrogen
•There is a tight fit between Ach and receptor binding site (little scope for variation).
•Methyl groups fit into small hydrophobic pockets. Van der wall interactions
•Ester interacts by hydrogen bonding (oxygen atoms are potential acceptors).
•Quaternary nitrogen interacts by ionic bonding.
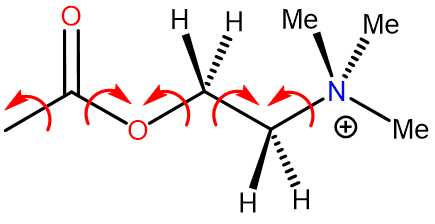
Acetylcholine conformations
Freely rotates around single bond
Large number of possible conformations with varying stability
Binds to different receptors
What are the two types of cholinergic receptors
Nicotinic: activates cholinergic receptors on skeletal muscle
Muscarinic: activates cholinergic receptors on smooth muscle and cardiac muscle
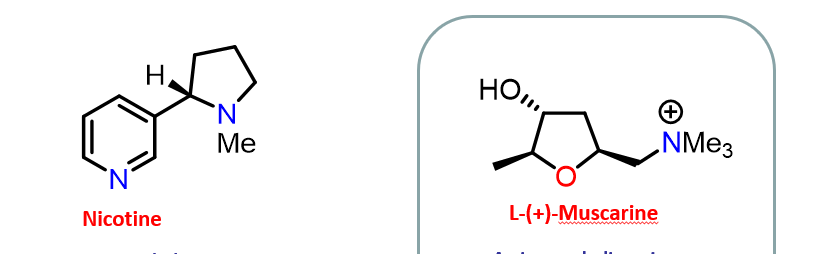
Importance of five membered ring
Ach unable to rotate, locked within a ring, rigid
Restricts the number of possible conformations
Requires separation of the functional groups from the rings
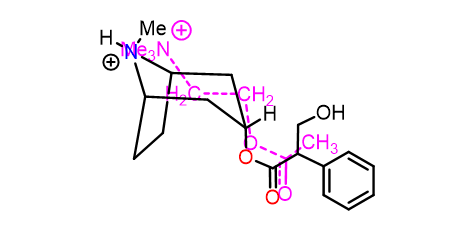
Atropine vs ACH
•Relative positions of ester and nitrogen similar in both molecules.
•Nitrogen in atropine in atropine must be protonated (ionised) when bound to the receptor.
•Amine and ester are important binding groups (ionic + H-bonds).
•Aromatic ring of atropine is an extra binding group (vdW).
•Atropine binds with a different induced fit (larger than Ach) - no activation.
•Atropine binds more strongly than acetylcholine as an antagonist
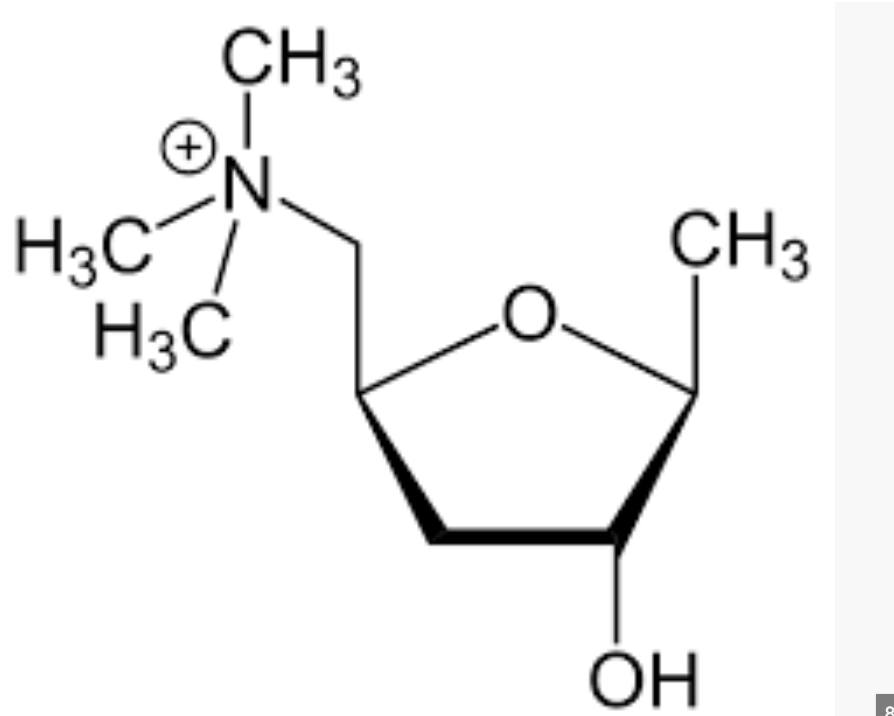
What is the effect of ionisation
Analogues are fully ionised
unable to cross BBB
avoids systemic side effects
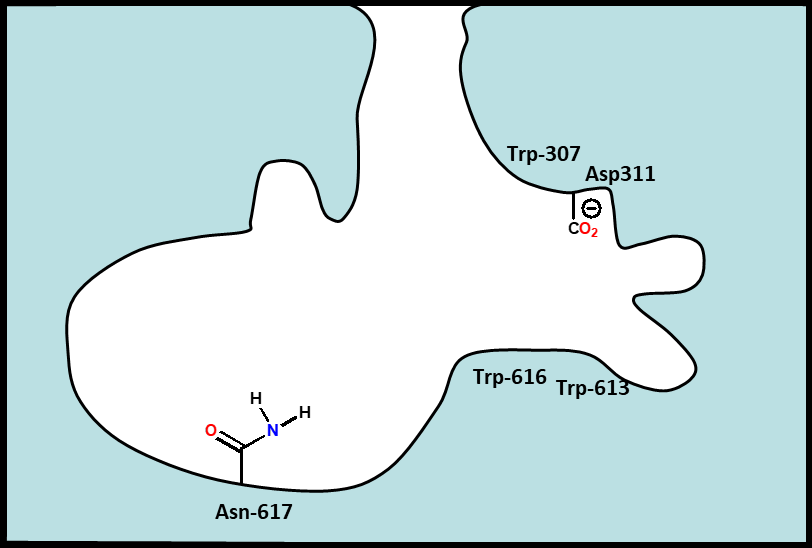
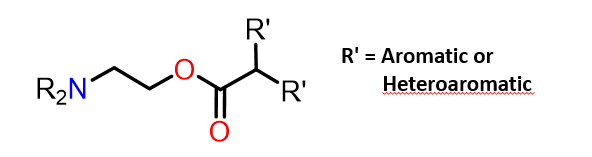
Describe the chemistry of muscarinic receptor antagonists
•Tertiary amine (ionised) or a quaternary nitrogen.
•Aromatic ring.
•Ester.
•N-Alkyl groups (R) can be larger than methyl (unlike agonists).
•Large branched acyl groups allowed (unlike agonists).
•R’ = aromatic or heteroaromatic ring.
•Branching at aromatic/heteroaromatic rings is important
Þ extra hydrophobic bind regions available next to normal Ach
binding site??
alpha-adrenoceptors
generally contracts smooth muscle (except gut)
B2 adrenoreceptors
Relaxes smooth muscle
B1 adrenoreceptor
Contracts cardiac muscle
B2 adrenoreceptors
Predominate in the airways
B1 adrenoreceptors
Predominate in the heart
Receptor-selective drugs
act selectively at different organs and tissues
Chemical messengers in adrenergic system
Catecholamines:
Adrenaline: hormone
Noradrenaline: neurotransmitter
Function of catecholamines
Adrenergic agonists, promote the release of chemical from a receptor
Catecholamine chemistry
•Phenol groups form H-bonds to binding site (especially b-adrenoceptors).
•meta-Phenol can be replaced with other hydrogen bonding groups.
•Alcohol forms a hydrogen bond to the binding site (R-enantiomer more active).
•Protonated amine forms an ionic interaction with the binding site.
•N-Alkyl groups affect target selectivity.
•Larger N-alkyl groups lead to selectivity for b-adrenoceptors.
•Aromatic ring forms van der Waals interactions with the binding site.
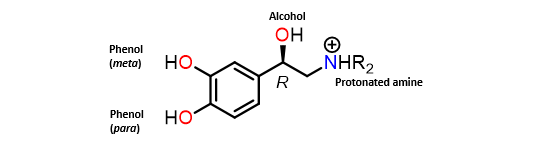
Breifly describe the adrenergic binding site
Hydrophobic pockets in B-receptors
Isoprenaline B-selectivity
•Shows some selectivity for b-adrenoceptors.
•Bulky isopropyl group introduces b-selectivity
(chain extension).
•BUT: no selectivity between b-subtypes.
•Cardiovascular side effects (b1 receptors).
Isoetharine selectivity
•Shows selectivity for b2-adrenoceptors.
•Ethyl group introduces b2-selectivity.
•Short lasting due to drug metabolism.
Metabolised by catechol-O-methyltransferase
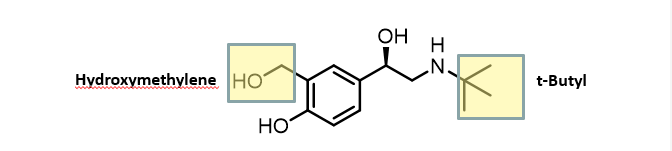
Salbutamol
B2- selectivety through hydroxymethylene group
Moving phenolic OH prevent metabolism but still has hydrogen bonding
Not cardioselective less side effects
duration of action 2-6h
A racemic mixture but R enantiomer is more active
SABA and SAMA
Ipratropium (SAMA = short-acting muscarinic antagonist)
Salbutamol (SABA = short-acting beta-2 agonist)