Module 5 - MR in Radiation Therapy Practice Questions
1/56
Earn XP
Description and Tags
RADTH 305 - MRI Physics. University of Alberta
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
give three acronyms of organizations involved in MR-RT
CADTH
Canadian Agency for Drugs and Technologies in Health
CPQR
Canadian Partnership for Quality Radiotherapy
ISMRM
International Society for Magnetic Resonance in Medicine
Name a cancer centre in Canada that currently has a MR-LINAC in use
Arthur J Child Comprehensive Cancer Centre
Princess Margaret Cancer Centre
Odette Cancer Centre
What is the MOMENTUM Study?
An international registry that collects clinical, imaging, technical, and patient reported data from patients treated with MR-guided radiotherapy to evalauate the safety, feasibility, and clinical benefit of MR-guided adapted radiation therapy and supporting evidence-based adoption of the technology.
Define margin reduction in the context of MR-RT
Margin reduction refers to decreasing the planning target volume (PTV) margins around the tumor because real-time MRI visualization, daily adaptive planning, and improved soft tissue contrast reduce set-up uncertainty, organ motion, and anatomical variation, allowing for more precise targeting while sparing normal tissue
Define dose escalation in the context of MR-RT
Safely increasing the radiation dose to the tumor by using real-time MRI guidance and daily adaptive planning to account for anatomical changes and motion, improving target coverage while maintaining normal tissue dose constraints
Define target delineation in the context of MR-RT
The process of accurately identifying and outlining the tumor and relevant target volumes (GTV, CTV, PTV) on imaging to ensure the prescribed radiation dose delivered to the intended area while minimizing dose to surrounding normal tissues
Define tumor tracking in the context of MR-RT
The continents or near-real-time monitoring of tumor position using MRI during radiation delivery, allowing the beam to be gated or adapted to tumor motion (ex. respiration) to maintain accurate dose delivery and reduce irritation of surrounding normal tissues.
List at least four differences between diagnostic MR and MR-Sim Equipment
patient positioning
MR-Sim allows treatment position using flat tabletops and immobilization devices whereas diagnostic MRI uses curved tables for comfort
RF Coils
Coil bridges are often used on patients to prevent deformity of patient outline
Geometric Accuracy
MRI systems prioritize geometric fidelity and distortion correction for RT planning, while diagnostic MRI prioritizes image contrast and diagnostic quality
Laser Systems
MR-Sim includes external laser alignment systems for reproducible patient setup and reference marks; diagnostic MRI does not
Comfort
diagnostic MRI has cushions on the treatment couch, MR-Sim does not as we are using immobilization devices
Bore Size
the bore in an MR-Sim is larger than diagnostic MRI so that immobilization devices can fit in machine
Describe the electron return effect in terms of it’s physics and RT significance
In MR-guided radiotherapy, the strong magnetic field causes secondary electrons produced by photon interactions to curve due to the Lorentz force. At tissue-air interfaces, electrons that would normally exit the patient are bent back toward the tissue, leading to increased electron fluence at the interface. The ERE results in dose enhancement at tissue-air interfaces (skin, lung, bowel gas) and potential dose reduction just beyond the interface, which can increase the risk of skin or interface toxicity and must be accounted for during treatment planning and dose calculation
what three factors affect the ERE
Direction of B0 relative to beam
magnitude and location of dose enhancement depends on orientation
Strength of B0 field
higher field strength causes greater electron deflection, increasing ERE severity
prescence and size of air cavities
ERE occurs in tissue-air interfaces, larger or more numerous gaps (lungs, skin) will increase effect
What two MR-LINACs are minimally affected by ERE and why?
Aurora-RT
electrons experience less lateral deflection as their velocity is aligned with the magnetic field
ViewRay MRIdian
lower field strength causes less deflection of secondary electrons
which MR-LINAC is most affected by the ERE
Elekta Unity
has a higher magnetic field strength and a B0 field oriented perpendicular to the photon beam, which causes greater lateral deflection of secondary electrons at tissue-air interfaces
give 5 main features of the ViewRay MRIdian system
6 MV Beam
0.35 T split superconducting magnet
magnet perpendicular to the beam
bore is 70 cm wide
couch moves in three directions
system is commercially available and there are many clinical publications on it
give 5 main features of the Aurora-RT System
6 MV beam
0.5 T biplanar Tc superconducting magnet with steel yoke
magnetic field is parallel to the photon beam
bore is 110 cm wide
couch moves in three direction
not commerally available with no clinical outcomes published
give 5 main features of the Elekta Unity System
7 MV beam
1.5 T closed superconducting magnet
magnet perpendicular to the beam
bore is 70 cm wide
commercially available and has many clinical outcomes
In the Elekta system, how is the system engineered so that the beam reaches the patient unobstructed by the magnet
The LINAC and gantry rotate around the MRI magnet in the Unity machine, and the photon beam passes through the magnet via a coil-free central gap in the magnet design. Specifically, the central 15 cm of the magnet contains no coils, creating a clear path for the radiation beam. This gap allows a maximum field size of approximately 24 cm in the head-to-toe (superior–inferior) direction at isocentre.
Although the beam passes through the magnet structure, the total material in the beam path is minimized and is equivalent to about 8.2 cm of aluminum, ensuring acceptable beam attenuation and dose delivery.
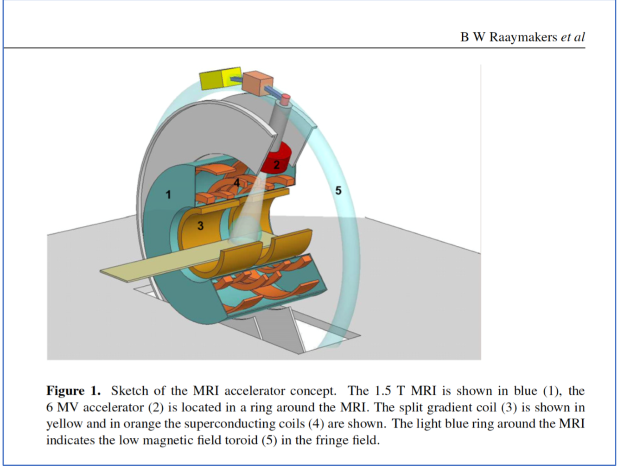
In the Viewray system, how is the system engineered so that the beam reaches the patient unobstructed by the magnet?
The MRI magnet is split into two halves, creating a central open gap at isocentre. The radiation beam passes directly through this gap rather than through the magnet structure, allowing it to reach the patient unobstructed and with minimal attenuation, while still maintaining the magnetic field required for MRI guidance.
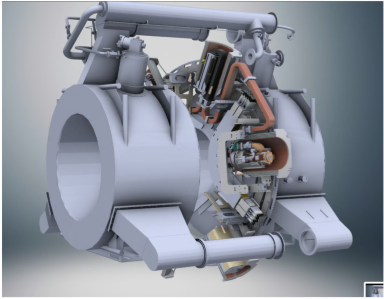
In the Aurota-RT system, how is the system engineered so that the beam reaches the patient unobstructed by the magnet
The MRI magnet is designed such that the B₀ magnetic field is always parallel to the photon beam. To achieve this, the magnet rotates together with the treatment gantry, ensuring that the beam path remains aligned with the magnetic field at all times. As a result, the radiation beam passes directly through the magnet without obstruction or attenuation, regardless of gantry angle, allowing continuous, unobstructed delivery of the prescribed dose.
Give the field strength and beam energies of 3 different MR LINAC Systems
ViewRay MRIdian: 6 MV beam and 0.35 T magnet
Elekta Unity: 7 MV beam and 1.5 T magnet
Aurora-RT: 6 MV beam and 0.5 T magnet
Australia MR-LINAC: 6 MV and 1.0 T magnet
Give the field orientation of three different MR LINAC systems
ViewRay MRIdian: split superconducting perpendicular
Elekta Unty: superconducting closed bore perpendicular
Aurora-RT: rotating superconducting parallel magnet
Australia: superconducting open bore parallel and perpendicular orientation.
The patient rotates in this machine!
Which was the first commercially available MR-RT Machine?
ViewRay MRIdian received FDA clearance in 2012. It combined a 0.35 T MRI scanner with a linear accelerator for real-time imaging and adaptive radiation therapy.
Name three disadvantages of CBCT vs. MRI for MRgRT applications
Poor Soft Tissue Contrast: limited differentiation between tissues, making it harder to visualize tumors/OARs
Limited Real Time Imaging/Motion Tracking: CBCT acquires images slowly and cannot continuously monitor tumor motion during treatment. Compare this to MRI that allows for real-time visualization and gating of moving targets
Higher Imaging Dose: CBCT uses ionizing radiation, MRI is non-ionizing
why is MRI particularly useful for proton therapy?
It provides superior soft-tissue contrast for accurate tumor and organ-at-risk delineation, which is critical in proton therapy due to its sharp dose fall-off (Bragg peak). Small errors in target definition or positioning can lead to significant underdosing of the tumor or overdosing of nearby normal tissue, so MRI’s high-resolution imaging helps maximize treatment precision and safety.
Describe a standard RT workflow without MRI
A typical radiation therapy workflow involves patient consultation, treatment planning using CT imaging, verification of patient positioning, administration of radiation doses, and follow-up assessments.

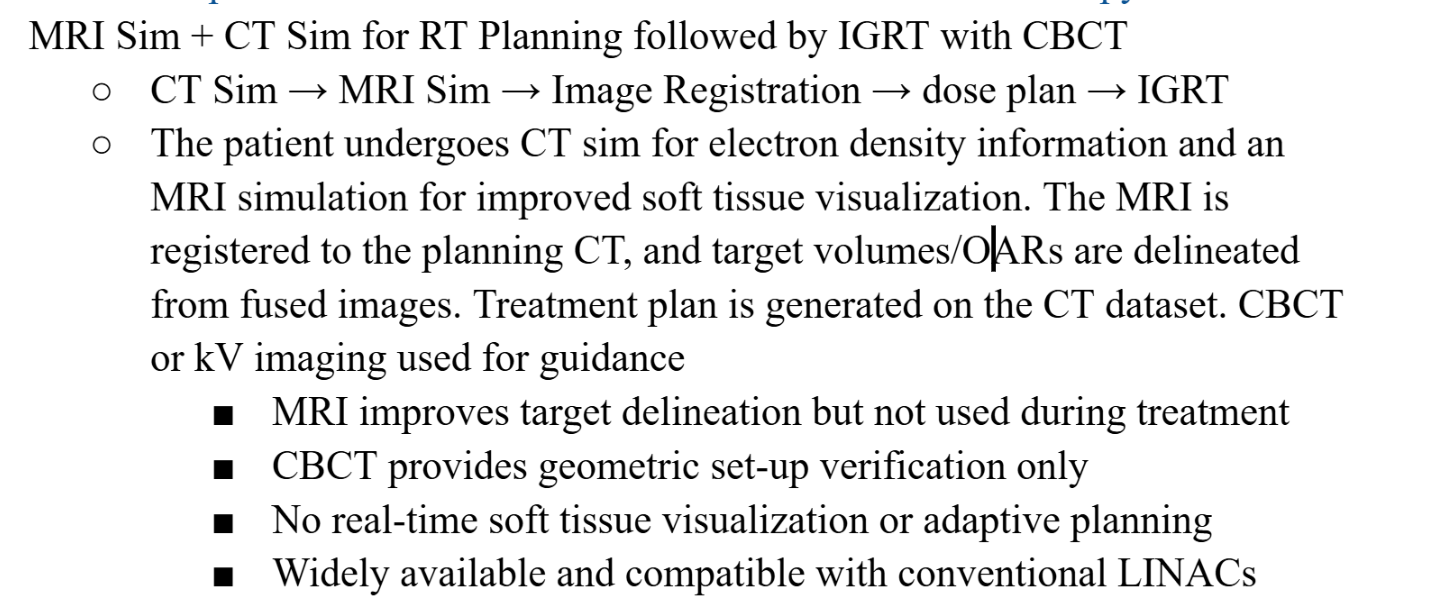
Describe the MR-CTgIGRT workflow
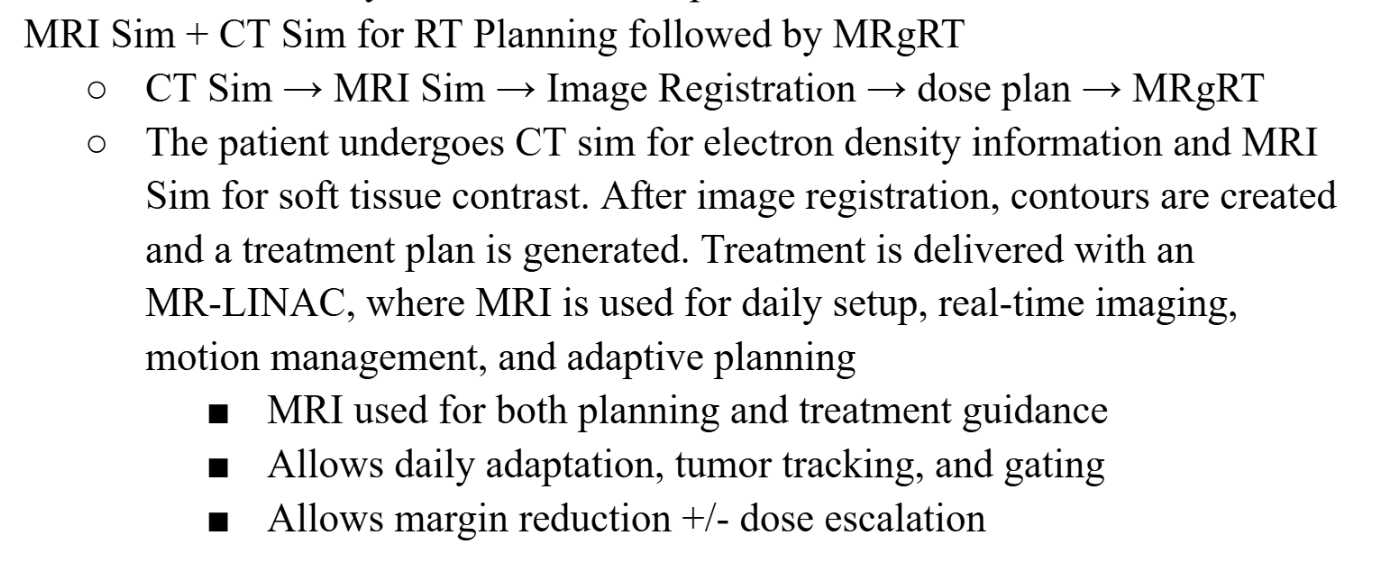
Descibe the MRgRT workflow
What are some pros of an MR-only workflow
improved target localization
real-time tracking/gating possible
normal tissue sparing
adaptive planning
fMRT= bioguided RT
decreased fractionation
what are some cons of an MR-only workflow
complications from tissue overdose
decreased dose rate (600 MU/min c.f 1200 MU/min)
slower gantry rotation
decreased field size
What is adaptive RT and when/who might it be useful for
ART is an approach in which the radiation treatment plan is modified during the course of the treatment to account for changes in patient anatomy, tumor size/shape, or organ motion using updated imaging. ART is particularly useful when there are significant anatomical or biological changes, such as tumor shrinkage/progression during tx, daily organ motion/deformation, weight loss or body contour changes, targets near critical organs where maintaining dose constraints is prioritized, and for pediatric patients who have rigorously regulated dose prescriptions to minimize long term effects
What does the term PseudoCT mean
A pseudoCT is a synthetic CT image generated from MRI data that assigns CT-like electron density (HU) information to tissues. This is how dose calculations are performed in MRI-only workflows, where conventional planning CTs are not acquired.
Define and Explain ATS
ATS (Adapt-to-Shape) is an online adaptive radiotherapy workflow used in MRgRT. It involves re-contouring the target and organs at risk on the daily MRI to reflect the patient’s current anatomy, followed by full reoptimization of the treatment plant before delivery. This accounts for anatomical changes such as organ deformation, tumor shrinkage, or variable filling. This ensures accurate target coverage while maintaining normal tissue dose constraints.
Define and Explain ATP
ATP (Adapt-to-Position) is an online adaptive radiotherapy workflow used in MRgRT. It involves shifting or re-optimizing the existing treatment plant based on the daily position of the target seen on MRI, without changing the target or OAR contours. The plan is adapted to account for rigid translational shifts and limited rotations while the shape of the anatomy is assumed to be unchanged.
When is ATP more useful? When is ATS more usful?
ATP is faster and less resource-intensive than Adapt-to-Shape (ATS) and is more useful when anatomy is stable but target position varies from day to day.
ATS is useful when shape and position of structures change significantly from day-to-day, but it is more time and resource-intensive than simpler adaptive approaches.
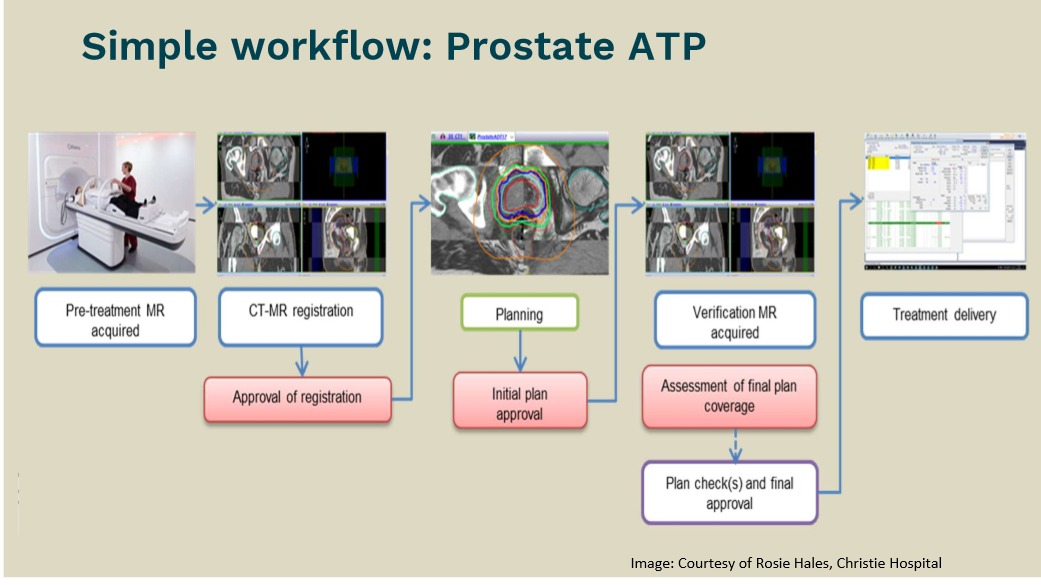
Draw a flowchart for an online prostate-ATP workflow
draw a flowchart for an online pelvic-ATS workflow
pretreatment CT taken to give electron density and a daily MRI is taken. They are combined in deformable registration so that the CT is adapted to the current anatomy seen on the MRI. Contours are then retraced based on today’s MRI and densities are applied to areas of concern (bladder). The online part is where the doses are replanned according to the combined CT/MRI data , then a conformational MRI and dose calcs are done for QA. Treatment is delivered at the same time as an MRI to ensure if the patient moves the dose is shifted. A post treatment is then done to confirm dose was delivered to the right spot

what is an imaging biomarker, and why is this of interest in MR-RT?
An imaging biomarker is a quantitative feature extracted from medical images (signal intensity, diffusion, perfusion parameters) that reflects biological processes, disease characteristics, or treatment response. In MRgRT, imaging biomarkers are valuable MRI acquired during treatment can monitor tumor responses, cellularity, or perfusion in real time. This enables response-adapted treatment like modifying the dose or margins to improve tumor control or minimize toxicities
MR is not sensitive to electron density (which is needed for treatment planning). How do MR-only workflows get around this?
MR-only workflows address this by generating a pseudoCT from the MRI, where tissues are assigned CT-equivalent electron density or Hounsfield unit values using methods such as atlas-based approaches, tissue classification, or machine learning. This synthetic CT enables accurate dose calculation without a planning CT needed.
What is the topic of AAPM TG-284
this task group reported on MRI simulation in radiotherapy. it provided recommendations for clinical implementation, optimization, and quality assurance of MR-Sim in RT. It includes guidance on equipment selection, siting, commissioning, workflow integration, motion and distortion management, safety, and imaging protocols tailored for RT planning
Explain three unmet needs in MR-SIM from the AAPM TG-284 paper
incomplete geometric distortion correction
While vendors provide 3D gradient nonlinearity (GNL) correction, it is not available for all imaging sequences (e.g., 2D, gated, DWI), may not be applied retrospectively, and residual distortions remain at large fields of view with no vendor-provided solution.
Limited B0 field mapping and correction
Online B₀ field mapping is often restricted to research options, may rely on wrapped phase images, and patient-specific online distortion correction is not clinically available.
Residual Intensity Non-Uniformity
Vendor correction algorithms reduce RF coil intensity variations but residual inhomogeneities remain, affecting image registration and segmentation; clinical correction tools are limited.
what are two major sources of image distortion in MRI
GNL: spatial distortion caused by imperfections in the gradient fields, which increases the distance from isocentre and affects geometric accuracy
B0I/Susceptibility: distortion arising from variations in the main magnetic field often due to patient anatomy or tissue-air interfaces, leading to spatial warping and signal loss
Discuss three QA issues that arise for MR in RT and how they are dealt with
Geometric Accuracy and Distortion:
MRI prone to GNL and B0 inhomogeneities which can compromise targeting accuracy
Addressed through vendor distortion corrections and phantom-based geometric QA
Image registration accuracy:
MRI is often registered to CT for dose calculation. Errors can affect target delineation
QA includes rigid/deformable registration checks, fiducial markers, and standardized imaging protocols
Magnetic Field Stability
Drift or inhomogeneity in the magnetic fields can degrade image quality.
Routine system QA, including field homogeneity checks and vendor-recommended calibration, is used to maintain stability.
Why is QA for MR so different than QA for X-ray machines
MRI QA focuses on image quality and geometric accuracy, not radiation dose or beam output. MRI images are affected by inhomogeneities and GNL, which cause distortion not seen in x-ray images. MRI errors are also sequence and patient dependent, unlike the more stable beam characteristics of x-ray machines.
Why/when is geometric distortion inevitable in MRI
Geometric distortion is inevitable in MRI because spatial encoding relies on magnetic field gradients and a highly uniform main magnetic field, which cannot be perfectly uniform over large DSV. distortion occurs farm from isocentre, at tissue-air interfaces, and with certain sequences (EPI, DWI), where GNL and B0I cause unavoidable spatial misregistration
Discuss how the choice of pulse sequence can affect geometric distortion
The choice of pulse sequence in MRI can significantly impact geometric distortion due to variations in echo times, gradient strengths, and the sensitivity to magnetic field inhomogeneities.
EPI and diffusion sequences have high distortion due to longer readout times and sensitivity to B0.
Shorter TE, FSE, 3D sequences generally produce less distortion than GE or EPI
Discuss how the choice of parameters can affect geometric distortion
higher receiver bandwidth reduces distortion, but the tradeoff is SNR
longer echospacing/readout increases distortion
larger FOV and off-isocentre images increase GNL
give at least three reasons to support the statement ‘MRI signal is not intrinsically quantitative’
The body interacts with the RF coils, so the strength of the RF signal is variable
RF coils are not uniform so transmit flip angle and receive sensitivity will vary
NMR system is not calibrated in uniform terms; we don’t know how many water molecules are in each voxel.
Images are weighted rather than being pure parameters
what 4 groups are represented within the CPQR
CPAC, CAMRT, COMP, CARO
compare spatial resolution requirements for MR-Sim vs. Diagnostic MR
image resolution needs to be higher for RT
In plane resolution = 1mm (this is fairly high for body, but normal for brain)
Slice thickness is 1-2 mm and 3 mm in body
Slice gaps are discouraged
Use more than one sequence to get more contrast
One image must encompass entire axial range of the targeted anatomy
If you are targeting the liver, you need to image the entire image plane of body that the liver passes through, will not need this in normal MRI
Give two reasons why 3D is preferred over 2D
3D image has more isotropic resolution
We have full volume of data so we can correct and rebuild distortions
3D uses two phase encoding gradients
Although 2D has good SNR, it has through plane distortion and slice imperfections
What is the minimum bandwidth readout required for MR-Sim for 1.5 T and 3T
1.5 T = 220 Hz/mm
3 T = 440 Hz/mm
readout bandwidth is increased to decrease geometric distortion
what material could you put in a phantom to make it useful for both MR and CT
A phantom that can produce tissue-like contrast on both MR and CT will contain different circles with various amounts of CaCO3. This will have adjustable T1, T2, and x-ray attenuation properties
describe a phantom to measure geometric distortion for MR
A geometric distortion phantom for MRI typically consists of a large, rigid structure with a known 3D grid of fiducial markers (e.g., spheres or rods) filled with MR-visible material and arranged at precisely known spatial locations across a large field of view. MR images of the phantom are acquired and the measured positions of the fiducials are compared to their known true positions (or to a CT reference), allowing quantification of spatial distortion as a function of distance from isocentre.
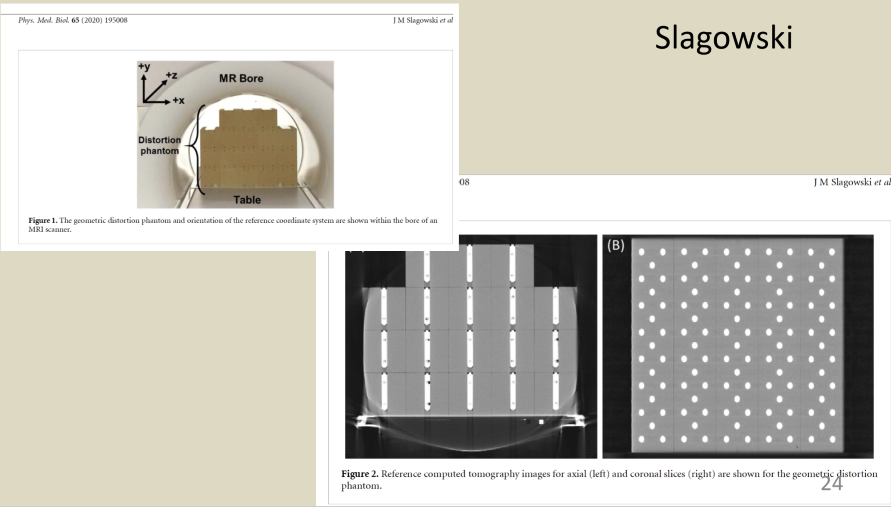
Describe 2D distortion correction
2D distortion correction is applied slice-by-slice and corrects in-plane distortion only. It does not account for through-plane errors, making it less accurate for RT applications, especially over large volumes
describe 3D distortion correction
3D distortion correction uses a full 3D model of gradient nonlinearity to correct distortion in all three spatial directions. It provides better geometric accuracy, particularly away from isocentre, and is preferred for MR-Sim and MRgRT.
why will be there less distortion away from the DSV in 3D compared to 2D
in 3D, we use two phase encoding gradients instead of just one. 2D also uses FE so you cann get major chemical shift issues.
we also have a full volume of data from 3D so we can use it to correct through plane distortions!
3T provides better SNR than 1.5 T, does this make it better for MR-Sim?
Not necessarily. While 3T will provide higher SNR than 1.5 T, it will be more susceptible to geometric distortion, susceptibility, and B0I which can compromise spatial accuracy. In MR-Sim, 1.5 T or even 0.35T systems are often preferred for having better geometric accuracy, even if SNR is lower.
Why is geometric distortion and artifacts worse in 3 T magnets over 1 T magnets?
Artifacts are worse at 3.0 T because 1 ppm is a larger frequency difference than 1 ppm on a 1.5 T magnet. We get more distortion per same ppm, thus susceptibility artifacts are worse in 3.0T magnets
3T magnet has a stronger field so it has stronger sensitivity to artifacts