nuclear chem exam
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
nuclear decay
process in which a unstable atomic nucleus transfers into a more stable one by emitting radiation
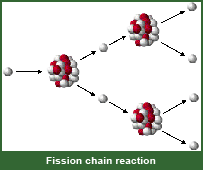
Explain how a nuclear chain reaction occurs with a drawing or words.
nucleus splits in two
form 2 new elements
purpose of neutrons in nucleus
Protons repel other protons bc theyre both positively charged.
Neutrons hold the protons together like glue.
Without the neutrons the nucleus becomes unstable since protons are trying to get away fom each other
how to find which element is more unstable
the element with the least amount of neutrons is the most unstable
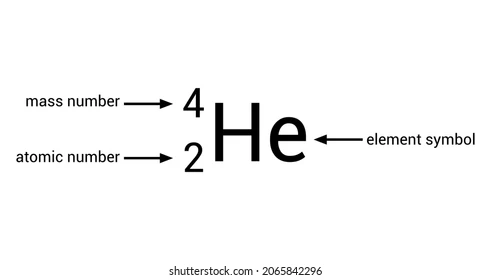
Alpha decay symbol
Beta decay symbol
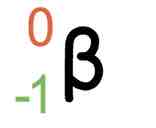
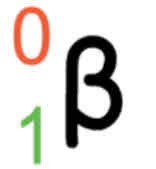
Positron emission
Electron capture
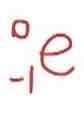
Gamma emission symbol
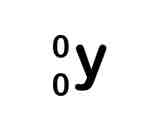
Adding protons symbol
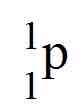
When using electron capture
The sides switch
Atomic mass formula
(mass #1 x abundance #1)+(mass#2 x abundance #2)
Do nuclear reactions follow the law of conservation of water
No,as then they would have to convert mass to energy
Name one specific imaging tool and what kind of nuclear decay is used
Scintgraphy:uses gamma radiation to make 2d images