orgo 2 exam 2 reagents and when to use them
1/80
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
81 Terms
ozonolysis of alkenes
1) O3
2)DMS
OR Zn, H3O+
Periodate cleavage of alkenes
1) OsO4, ether and NaIO4, H2O
oxidation of primary alcohols
PCC
oxidation of secondary alcohol
PCC or CrO3, pyridine
Friedel-Crafts Acylation
1) ClCOR, AlCl3
2) H3O+
formation of cyanohydrin
HCN, OH- catalyst
** think that killing something with cyanide is basic af, so thats why this rxn takes place in basic conditions)
cyanohydrin to hydroxyamine
H2, Ni
cyanohydrin --> hydroxycarboxylic acid
NaOH/H2O OR H3O+
formation of gem-diol(hydrate)
H2O; H+ or OH-
reduction of aldehydes to primary alcohols
r1
option 1) NaBH4, H2O in EtOH
option 2)
1) LiAlH4, ether
2) H3O+
reduction of ketones to secondary alcohols
r2
option 1) NaBH4, H2O in EtOH
option 2)
1) LiAlH4, ether
2) H3O+
reduction of carbonyl by catalytic hydrogenation
H2, Pd/C
Clemmenson Reduction
Zn(Hg), HCl
*Think that it sounds like clementine and it is zesty to Zn(Hg) because you feel like u are on mercury with that zestiness*
oxidation of hydrate from aldehyde
1. KMnO4, OH-
2. H3O+
*KMnO4 sounds to cnty so its a fem queen and turns the CHO into a COOH**
oxidation of hydrate from ketone
NR
Baeyer-Villiger reaction, also cyclic ketone to lactone
RCO3H, MCPBA or other peracid
hemiacetal formation
Use acids like H2SO4, TsO4, HCl with 1 equivalent of ROH
acetal formation
Use acids like H2SO4, TsO4, HCl with 2 equivalents of ROH
acetal hydrolysis
add H3O+ and just protonate and deprotonate from there until you get to a hemi, and then a carbonyl and alcohol
vinyl ether to acetal
EtOH, H2SO4, acidic conditions
protection of OH using vinyl ether
use vinyl ether like DHP in basic conditions(like TsOH), add H3O+ and heat to hydrolyze acetal and get alcohol back
protection of C=O using thiol
use HS-(CH2)x - SH with ZnCL2, Et2), do reactions
hydrolyze product with HgCl2, CH3CN, H2O and CaCO3 to get carbonyl group back
C=O to CH2 via thioacetal
sh-(ch2)3-sh with ZnCl2 to form thioacetal
raney ni to hydrolyze thioacetal along with the corbonyl that once existed
cyclic hemiacetal
H+
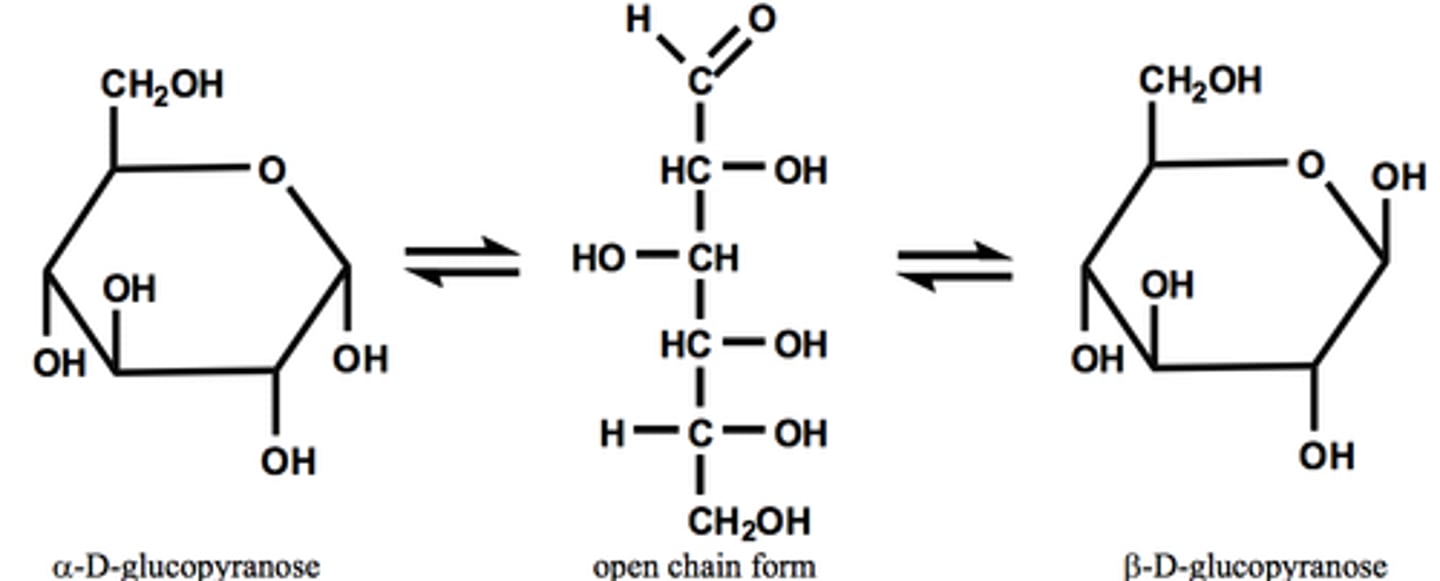
open chain to pyranose
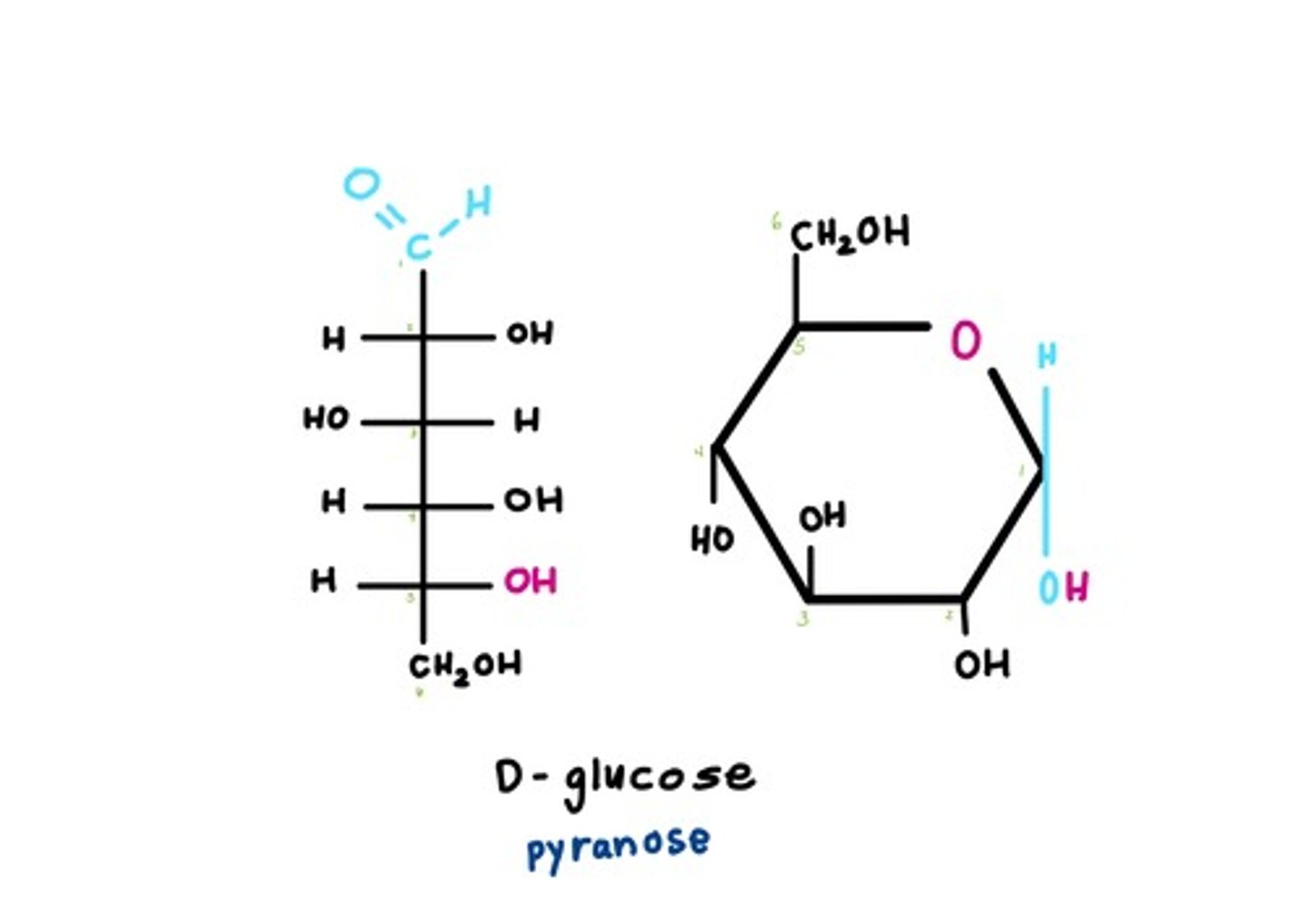
open chain to furanose
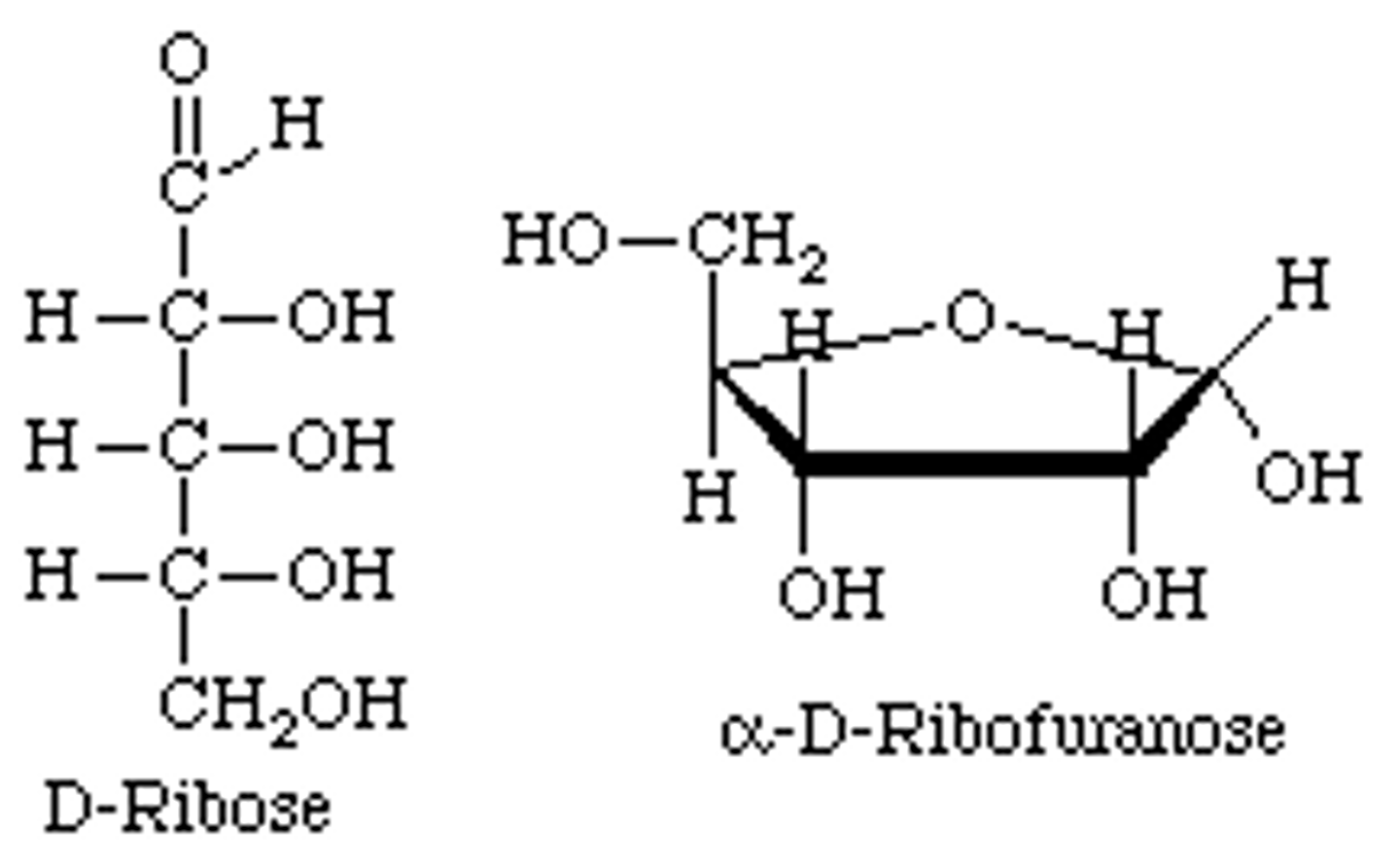
mutarotation
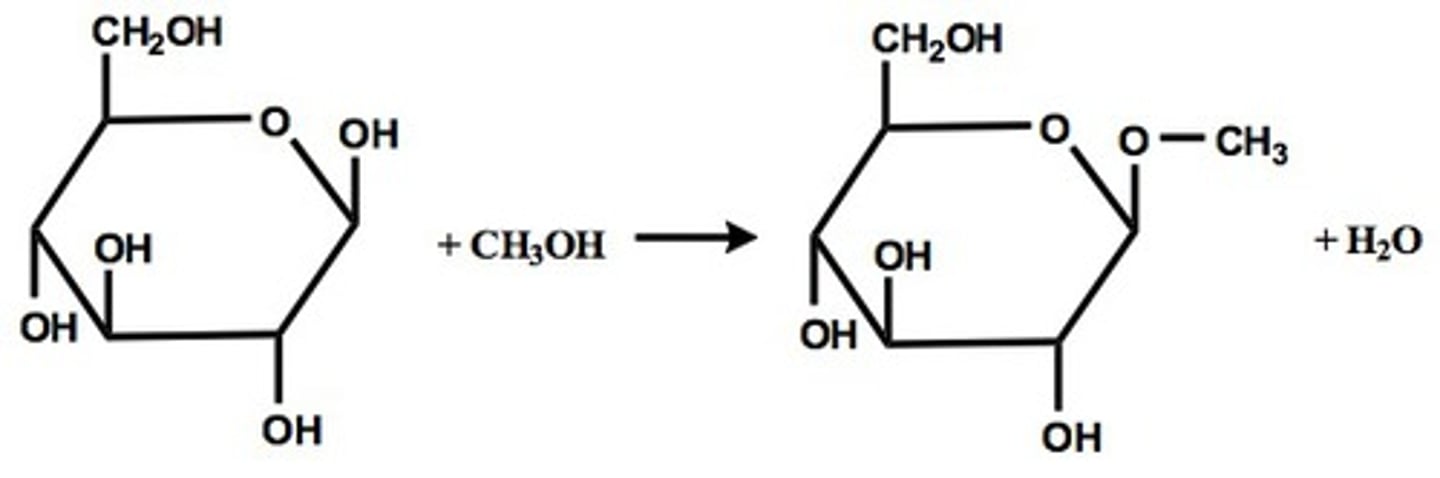
glycoside formation
acid catalyzed; product is acetal
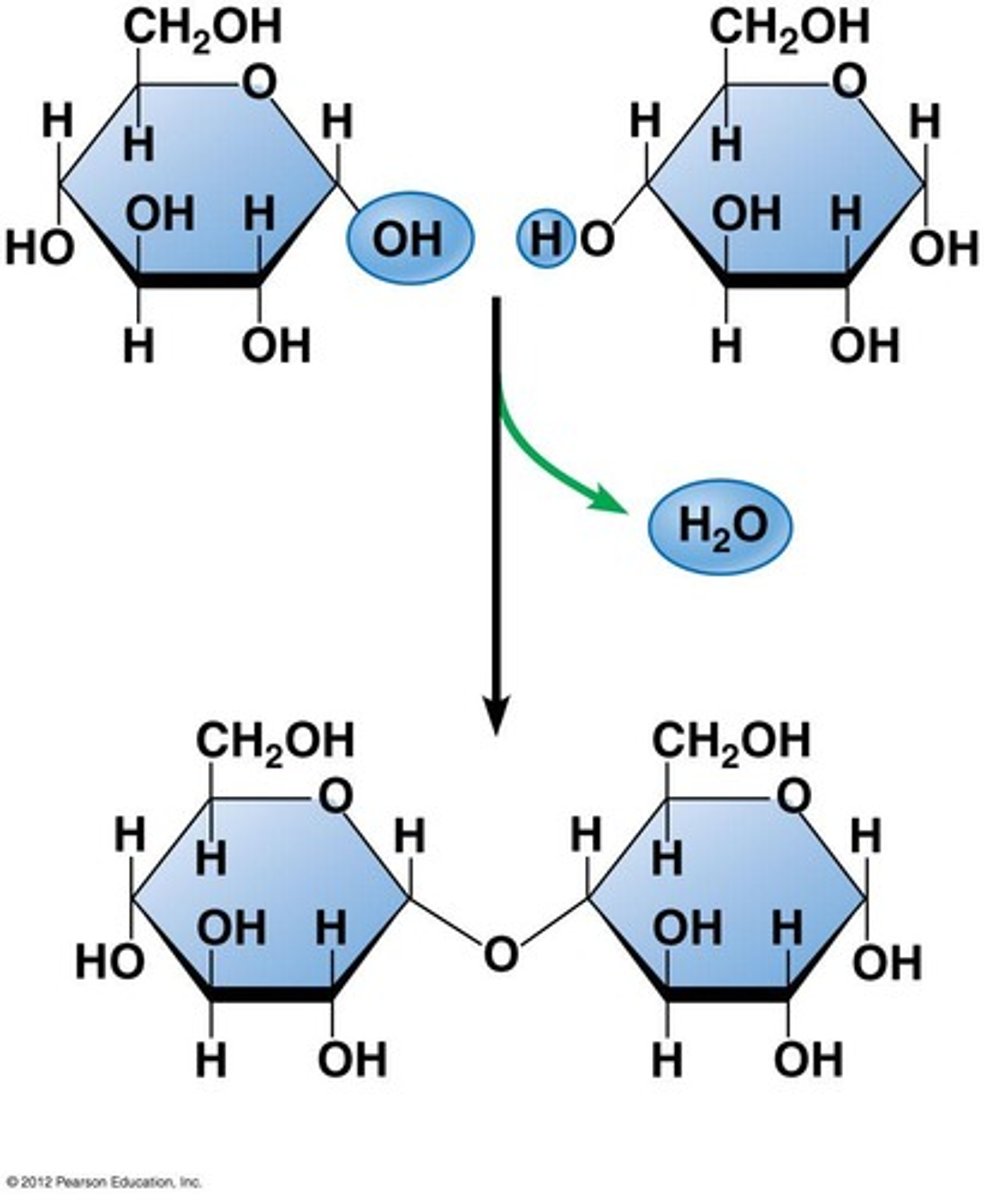
formation of disaccharide
h2o removed
reduction of aldose
-NaBH4, H2O, EtOH; can also use H2/Ni
-alditol
reduction of ketose
-NaBH4, H2O, EtOH
forms 2 epimeric alditols
oxidation of reducing sugars
Fehling's: CuSO4(aq), potassium tartrate, NaOH heat
Tollen's:[Ag(NH3)2]+-OH
*reacts as open chain; acetals can't be reducing sugars
enediol rearrangement
OH- then H2O because oxidization of aldose drives equilibrium to the right
oxidation to form aldonic acids, which can exist as lactones
Br2, H2O in acidic conditions
oxidation to form aldaric acids
HNO3; both CHO and CH2OH at end are oxidized and can be meso
imine formation
R-NH3, H+
imine hydrolysis
H3O+
hydrazone formation
H2N-NH2, H+
oxime formation
HO-NH2
enamine formation
secondary amine, H+, water to deprotonate alpha hydrogen and change double bond from C=N to C=C after imine intermediate
hydrolysis of enamines
H3O+
Wolf-Kishner Reduction
H2N-NH2, KOH, heat
reduction of N-containing groups
R-NH2, NaBH3CN, EtOH, H2O, acidic conditions
enamines as nucleophiles
react with alkyl halides via sn2 to form iminium ions; iminium ions via hydrolysis to form alpha alkyl/acyl ubstituted aldehyde or ketone
reduction of enamine
tertiary amine formed; H2, Pd/C reduces alkene part
Wittig reaction
PPh3 + Cl-R --> phosphoylide(carbanion)
Horner Emmons Reaction
1. P(OMe)3; 2. NaOEt, EtOH
beckmann rearrangement
H2SO4, H2O and heat
oxidation of aldehydes/primary alcohols
H2CrO4 or Na2Cr2O7, H2SO4 or
1. Ag2O, NH3
2. H3O+
oxidation of primary alcohols
1. KMnO4/OH-
2. H3O+
oxidation of alkylbenzene containing benzylic hydrogens
1. KMnO4/OH-
2. H3O+
hydrolysis of nitriles
first get good leaving group on reactant
1. NaCN, DMF
2. H3O+ and heat
carboxylation of Grignard reagents
1. form grignard reagent with Mg in polar aprotic solvent
next
1. CO2
2. H3O+
formation of acid chloride
react SOCl2 with thionyl chloride, then Cl- ion, rearrange to form RCOCl
acid chloride to acid anhydride
-O-COR
acid chloride to ester
R-O- or ROH/pyr
acid chloride to amide
R-N^-H or excess R-NH2
acid chloride to carboxylic acid
H2O, pyr
acid chloride to carboxylate
N/A
ester to carboxylic acid(acid catalyzed ester hydrolysis)
H2O, HCl
saponification(ester to carboxylate)
1. OH, heat
2. acid workup
ester to different ester(transesterification)
R-O- or ROH/H+
ester to amide
R-N^-H or R-NH2/H+
anhydride to carboxylic acid
+H2O
anhydride to ester plus carboxylic acid
R-O- or ROH
anhydride to amide
R-N-H or excess R-NH2
amide to carboxylic acid
H3O+, heat
amide to carboxylate
OH-, heat
hydrolysis of nitriles(acid catalyzed)
H3O+
hydrolysis of nitriles(base catalyzed)
OH-
reduction reactions of acid chloride(RCOCL), ester(RCOOR), carboxylic acids(RCOOH)
1. LiAlH4, ether
2. H3O+
reduction of amide to amine
1. LiAlH4
2. H3O+
3. OH-
reduction to of acid chloride to aldehyde
Lithium tri(tertbutoxy)aluminum hybride
reduction to of ester to aldehyde
DIBALH and hydrolysis with H3O+
acid chloride addition elimination with organmetallic
R-MgX to form tertiary alcohols
R2CuLi to form ketones
ketones do not react with organocuprates
and acid workup
esters
RLi or R-MgX to form tertiary alcohol
formate esters with RLi and MgX to form secondary alcohols
and acid workup
tertiary amides
Grignard, polar aprotic and acid workup
nitriles
grignard and acid workup
Fischer esterification
ROH, H+
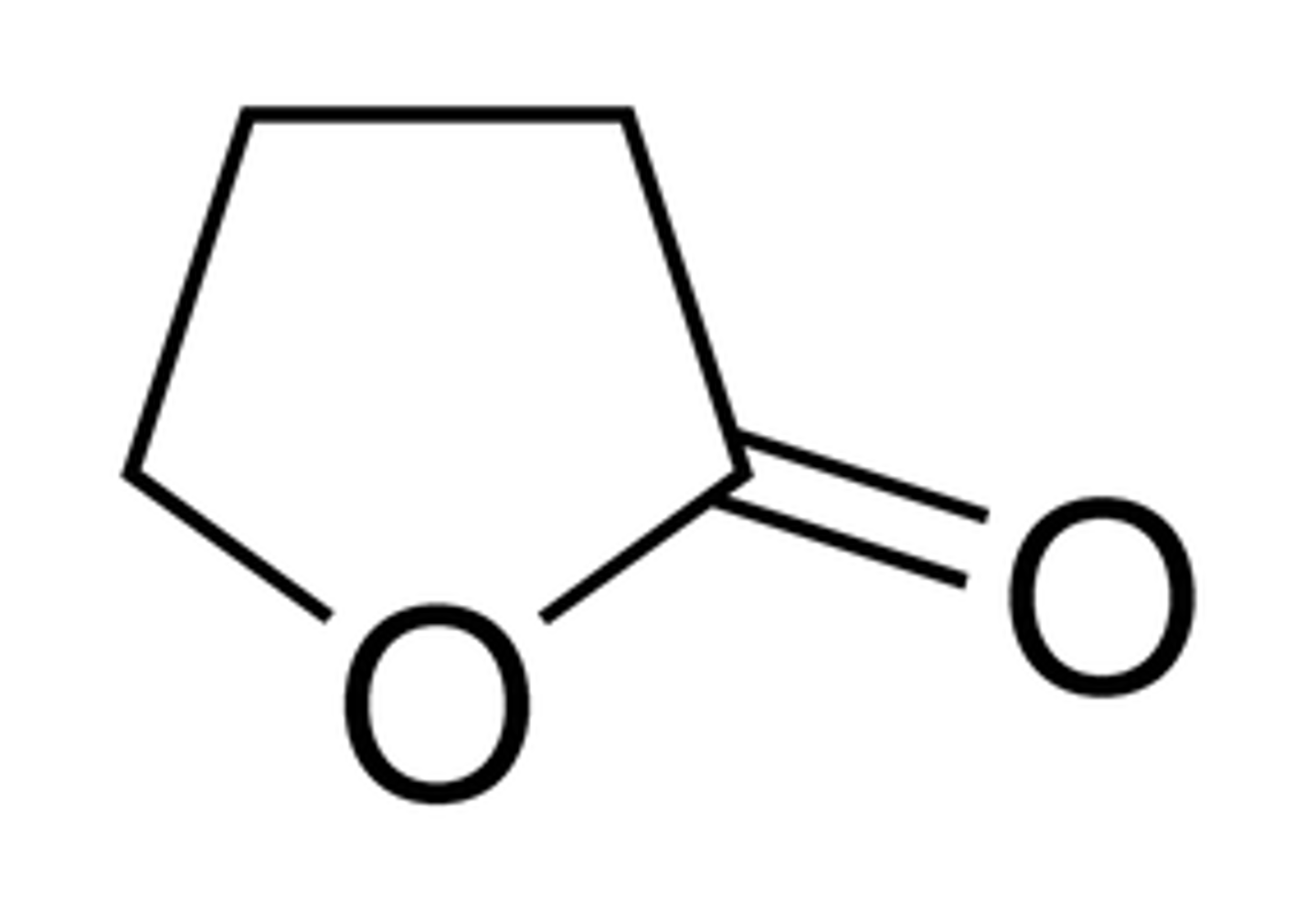
lactone formation
H-X, X-
or Br2/H2O in acidic conditions
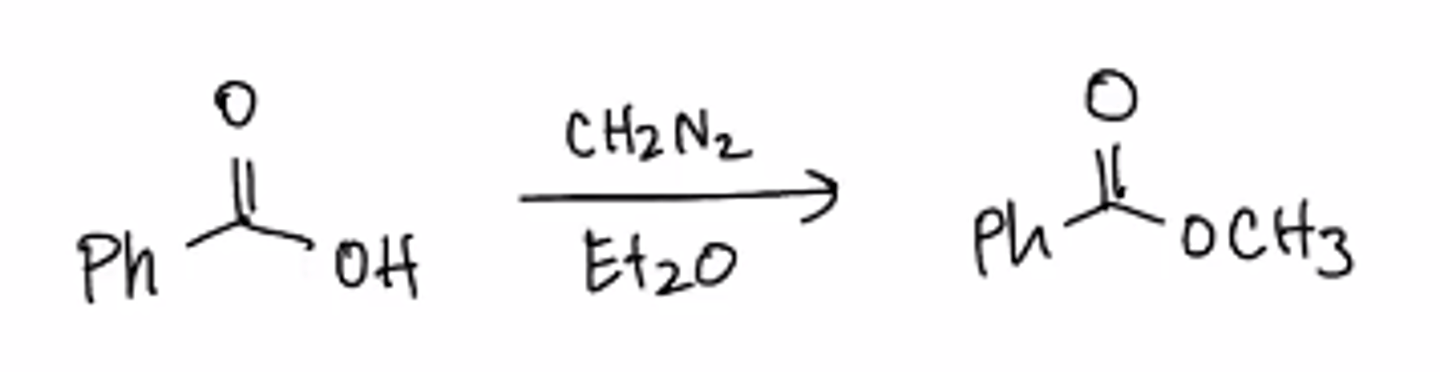
formation of methyl ester
diazomethane, ether