B1.2.9 Dependence of tertiary structure on hydrogen bonds, ionic bonds, disulfide covalent bonds and hydrophobic interactions
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
Tertiary structure
s the folding of a whole polypeptide chain into a three-dimensional structure. This structure is stabilized by interactions between R-groups.
4 main types of interaction
Ionic bonds
Hydrogen bonds
Disulfide bonds
Hydrophobic interactions
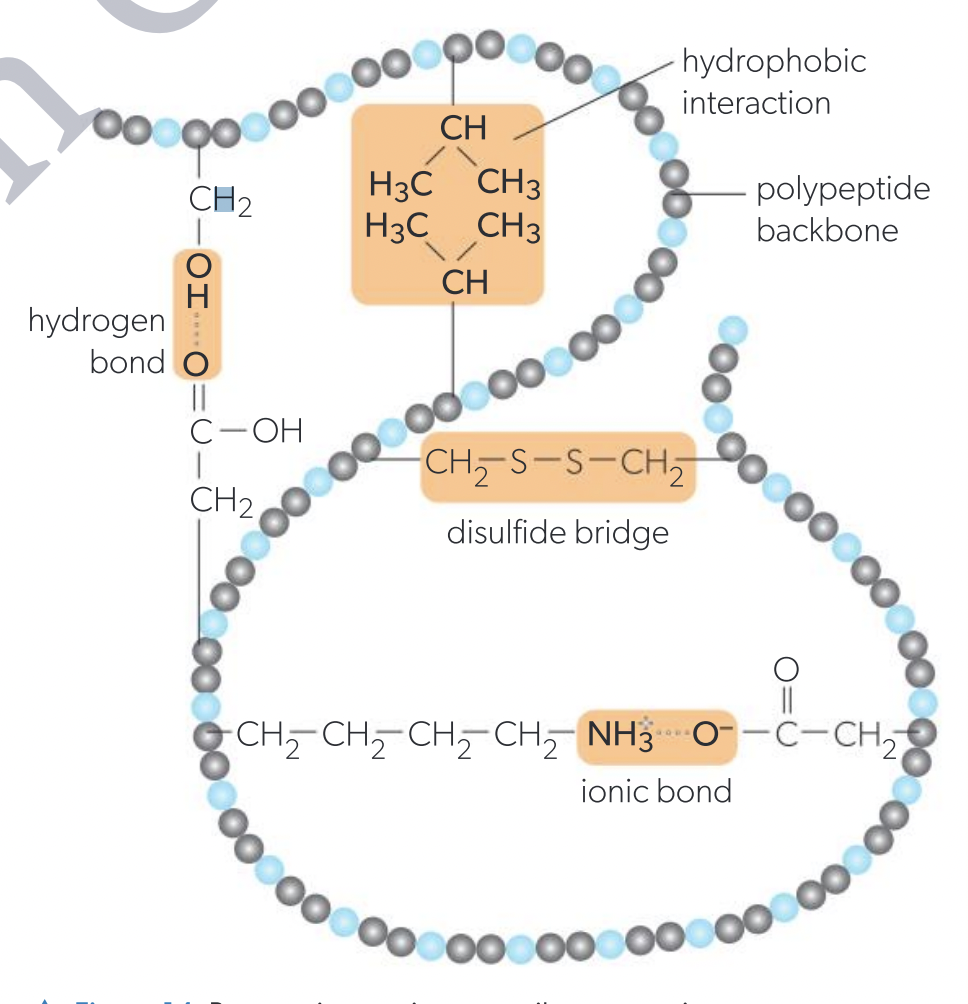
Ionic bonds
Between positively and negatively charged R groups
Amine groups become positively charged by accepting a proton ( NH2 + H + → –NH3+ ).
Carboxyl groups become positively charged by donating a proton ( COOH → –COO + H + ).
Because of the involvement of protons (hydrogen ions), ionic bonds in proteins are sensitive to pH changes
Hydrogen bonds
Between polar R groups
A hydrogen atom forms a link between two electronegative atoms such as O or N. It is covalently bonded to one of them, which results in the hydrogen having a slight positive charge, making it attractive to the other, which has a slight negative charge.
Disulfide bond
between pairs of cysteines. This is a covalent bond and the strongest of all the interactions.
Hydrophobic interactions
Hydrophobic interactions between any of the non-polar R-groups.
Tertirary structure
Tertiary structure develops as a polypeptide is synthesized by the ribosome.e. In some cases, a chaperone protein helps with this process to ensure that it results in a correctly folded and fully functional protein.
Chaperone proteins
Chaperone proteins, or molecular chaperones, are proteins that assist others to fold properly during or after synthesis, to refold after partial denaturation, and to translocate to the cellular locales at which they reside and function.
Wide range of 3d structures produced
A wide range of three-dimensional shapes is produced, most of which are globular. Within these tertiary structures there are often parts with secondary structure—α-helices and/or β-pleated sheets.