extraction of iron from hematite
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
1
New cards
hematite
irons ore
2
New cards
bauxite
aluminiums ore
3
New cards
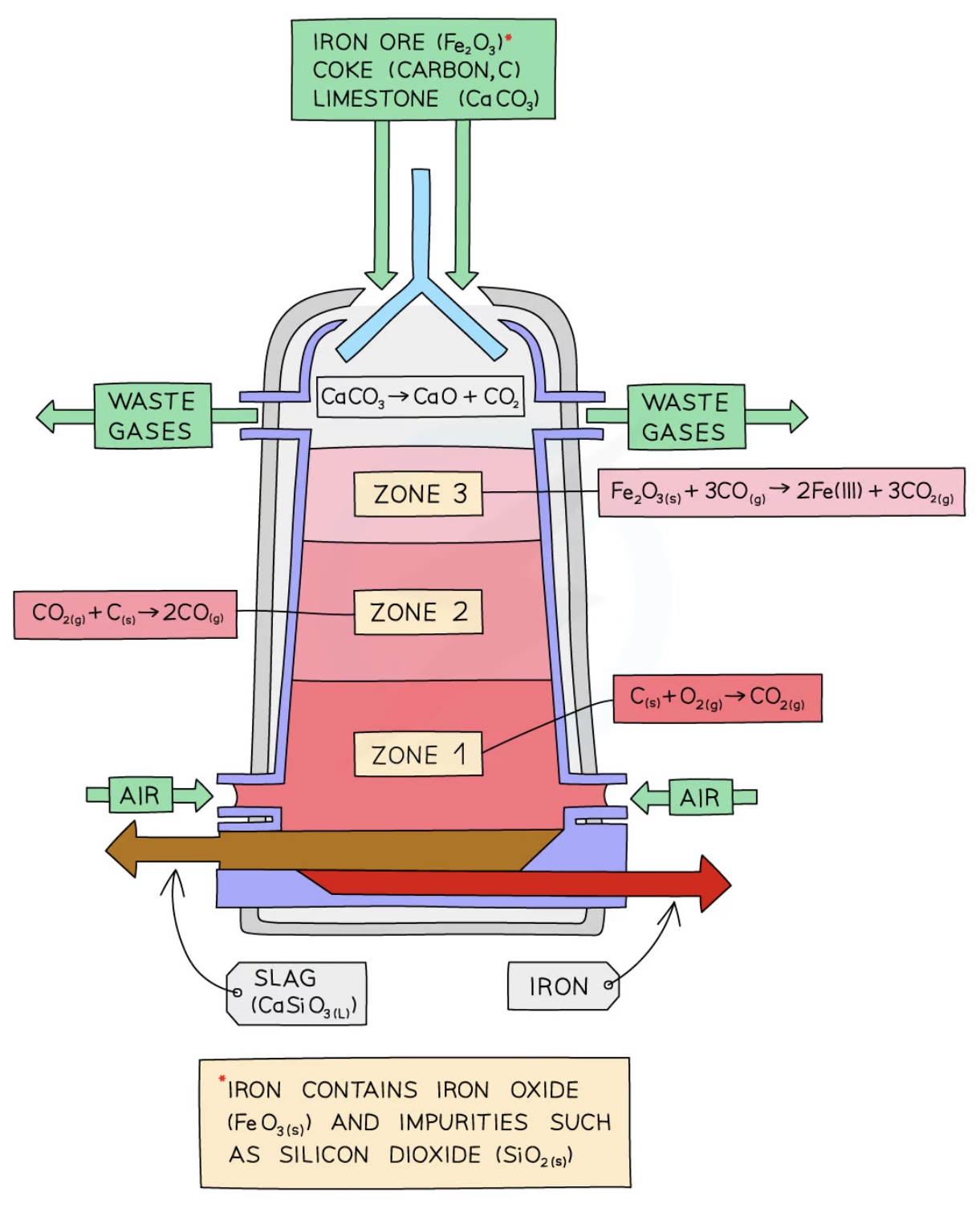
blast furnace
4
New cards
coke
an impure form of carbon
5
New cards
step 1
Coke burns in the hot air forming carbon dioxide
The reaction is exothermic so it gives off heat, heating the furnace
carbon + oxygen → carbon dioxide
6
New cards
step 2
At the high temperatures in the furnace, more coke reacts with carbon dioxide forming carbon monoxide
Carbon dioxide has been reduced to carbon monoxide
carbon + carbon dioxide → carbon monoxide
7
New cards