Chemistry - Unit 1+2
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
Matter
Any substance that occupies space and has mass
Mass
Amount of matter an object contains
Volume
Amount of space an object occupies
Pure substance
Matter that has the same composition throughout
Element
Simplest form of matter with unique properties
Compound
Substance with 2 or more elements has its own properties
Law of Conservation of Mass
Mass is not created or destroyed during a physical or chemical change. Mass can only be converted from one form to another

Chemical Symbol
A way of representing an element with symbols

Chemical Formula
A way of representing a compound with symbols
Mixture
Physical combination of of 2 or more substances
Heterogeneous mixture
Mixture that is not uniform throughout
Homogeneous mixture
Mixture that is the same throughout
Solution
A name for homogeneous mixture, usually a liquid
Solute
The substance being dissolved
Solvent
The substance doing the dissolving
Solid
Matter that has a fixed shape and volume
Liquid
Matter that has a fixed volume but no fixed shape
Gas
Matter that has no fixed shape or volume
Aqueous Solution
Solution where the solvent is water
Plasma
Superheated matter to the point electrons are pulled from atoms
Freezing point
Liquid state to a solid state
Melting point
Solid state to a liquid state
Boiling point
Liquid state to a gas state
Condensation point
Gas state to a liquid state
Sublimation
Solid state turning directly to a gas state
Deposition
Gas state turning directly to a solid state
Physical property
Property observed by a substance without changing the composition
Physical Change
Change to a substance that does not change composition
Examples of physical change
Does not create a new substance, changes in size, shape, or state
Chemical Property
Ability for a substance to undergo a chemical reaction
Chemical Change
Change that changes the composition of a substance
Example of chemical change
A new substance is formed, bubbling, releasing gas, precipitate form, flammability, corrosion
Chemical Reaction
1 or more substances that produce 1 or more product
Precipitate
Solid that forms from the mixing 2 or more liquids
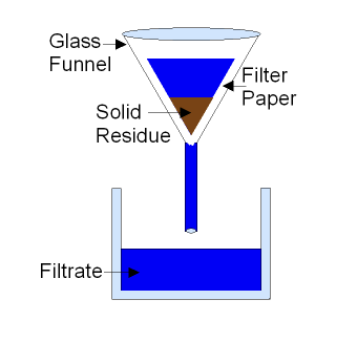
Filtration
Separate solid from liquid in heterogeneous mixture

Decantation
Separate 2 liquids with different density
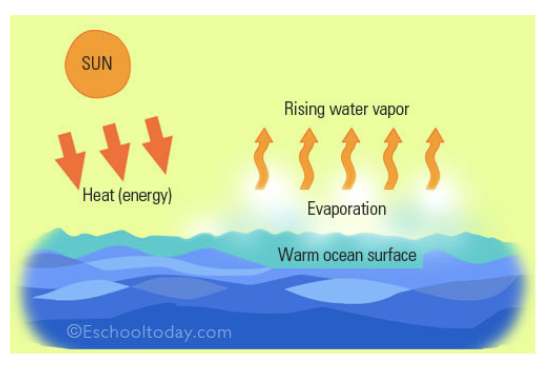
Evaporation
Separate dissolved solid from liquid; keep only solid
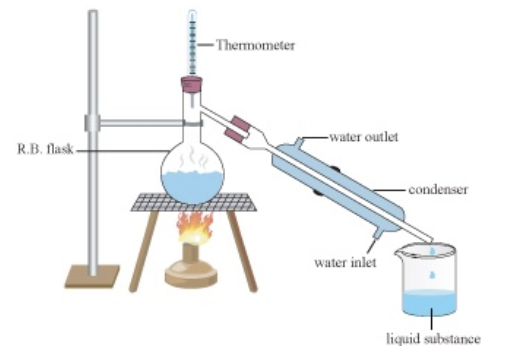
Distillation
Separate dissolved solid from liquid; keep both solid and liquid
Desalination
A process that removes mineral components (salt) from saline water
Scientific Method 1
Observation
Scientific Method 2
Hypothesis
Scientific Method 3
Experiment
Scientific Method 4
Analyze/Make conclusions
Scientific Method 5
Retest (experiment)
Hypothesis
proposed explanation made on the basis of limited evidence as a starting point for further investigation
Theory
Well experimented answer to an observation
Law
Mathematically supported answer to an observation; always correct