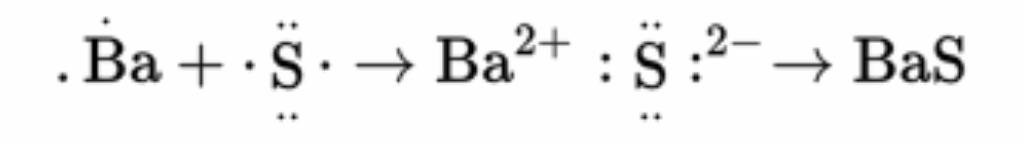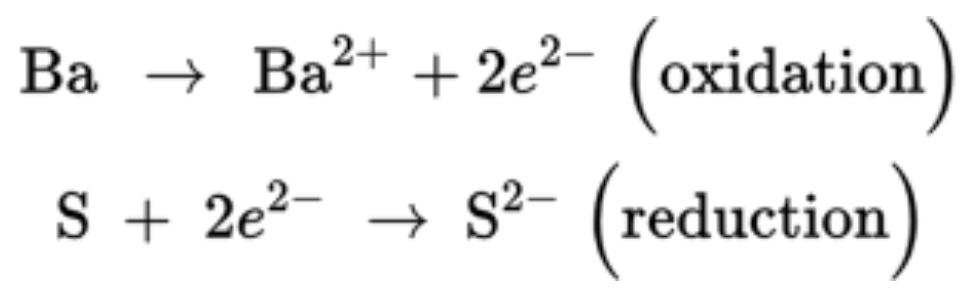Chapter 12: Oxidation-Reduction Reactions
Oxidation-reduction reactions (redox) occur when electrons are transferred from one atom to another
Oxidation is a loss of electrons
Reduction is the gain of electrons
When barium reacts with sulfur, the following reaction occurs:
The barium loses the two valence electrons and is oxidized. The sulfur gains the electrons and is reduced.
The same reaction can be written as two half-reactions to show each oxidation and reduction steps
Oxidation Numbers
Determining Oxidation Numbers
- To find if an element has transferred electrons, the oxidation number (or oxidation state) is calculated
- Oxidation Rule Hierarchy
- The oxidation numbers of all atoms add up to the charge of the atom
- Oxidation number for alkali metals is +1, alkaline earth metal charge is +2, and metals in group IIIA is +3
- Hydrogen oxidation is +1, fluorine is -1
- Oxygen is -2
- Halogens are -1
- Group VIA is -2
- Rule 1 is most important and rule 6 is least important. If two rules conflict, the higher one is obeyed
- Examples
- For CaCl2
- According to rule 2, calcium is +2. According to rule 5, each chlorine is -1. Most importantly, rule 1 indicates the oxidation state of this compound MUST be zero since the charge is zero.
- Adding up the charges: (+2) + 2(-1) = 0
- For KOH
- Rule 2 states potassium is +1. Rule 4 states oxygen is -2. Rule 3 state hydrogen is +1. Rule 1 is followed when adding up the charges:
- (+1) + (-2) + (+1) = 0
- Rule 1 can be written as an equation
- Total charge = (ox. no. 1)(subscript 1) + (ox. no. 2)(subscipt 2) + …
- Example
- For HClO4
- hydrogen is +1, chlorine is -1, -2 for each oxygen. However, this disobeys rule 1 because these would add up to -8 when it needs to be zero, so rule 5 is ignored
- 0 charge = (ox.no. H)(1) + (ox.no. Cl)(1) + (ox.no. O)(4)
- 0 = (+1)(1) + (ox.no. Cl)(1) + (-2)(4)
- ox.no. Cl = +7
Using Oxidation Numbers
- The change in oxidation states determines how many, or if, an exchange if electrons has occurred
- When permanganate reacts in an acid solution to form Mn+2, the oxidation number of manganese is +7 in the permanganate ion. This shows a redox reaction has taken place. More specifically, it has been reduced by gaining 5 electrons
Balancing Redox Reactions
- Ion-electron method for balancing reactions. step 1-6 are for acid solutions (H+). Step 7 is adding only if the reaction occurs in basic solution (OH-) or H+ appears on one side and OH- on the other:
- Write two half-reactions, one for ox and the other for reduc
- Balance all atoms in the half-reactions except for H and O
- Balance oxygen in half reaction by adding one H2O for each oxygen needed.
- Balance hydrogen by adding H+
- Balance the charges by adding the proper numbers of electrons. Electrons should be added to left side on one half reactions and the right side on the other half reaction
- Multiply each half reaction so they each have the same number of electrons. Add the half reactinos to electrons cancel out, also common ion cancels out. Simplify coefficients
- Add one OH- for each H+ ion to both sides of the equation in step 6. Combine H+ and OH- to H2O and cancel molecules on both sides and simplify if possible
- For the reaction I- + IO3- → I2
- I- → I2 and IO3- → I2
- 2I- → I2 and 2 IO3- → I2
- 2I- → I2 and 2 IO3- → I2 + 6 H2O
- 2I- → I2 and 12H+ + 2 I-3 → I2 + 6 H2O
- 2I- → I2 + 2e- and 10e- + 12H+ + 2 IO3- → I2 + 6 H2O
- 10 I- → 5 I2 + 10e- and 10e- + 12 H+ + 2 IO3- → I2 + 6 H2O
- Adding equations: 10 I- + 10e- + 12 H+ + 2 IO3- → 5 I2 + 10e- + I2 + 6 H20
- Canceling out: 5 I- + 6 H+ + IO3- → 3 I2 + 3H2O
Common Redox Reaction
Single Replacement (Displacement) Reactions
- Single replacement is when an atom in a compound replaces another atom and produced another element and a new compound
- Zn + 2 HCl → ZnCl2 + H2
- Active metals can react with water in single-displacement reactions
- Li, Na, K, Rb, Cs, Ca, Sr, Ba
- Active metals that do not react with water but will react with acid in single-replacement
- Mg, Zn, Pb, Ni, Al, Ti, Cr, Fe, Cd, Sn, Co
- Inactive metals do not undergo simple single-replacement with water or acid
- Ag, Pt, Au, Cu
- Activity series lists metals in order of strengths to cause redox reactions
Electrochemistry
- Redox reactions that would not normally occur but can occur by adding electricity in an electrolytic cell
- Spontaneous redox reactions that occur without added energy create a flow of electrons in galvanic cells
Electrolysis
- Electrolysis experiments would not normally occur but add two electrodes in electrically conductive sample and the voltage is adjusted until the electrons flow from electrodes
- One electrode is the cathode, where the electrons are supplied
- The anode electrode causes oxidation reactions to occur
Quantitative Electrochemistry
- Faraday’s constant (F) is usede to convert 1 mole e- to coulombs
- 1 mole e- = 96,485 coulombs
- moles of e- = It / F
- I is coulombs per second
- Time is units of seconds
- Faraday’s constant
Galvanic Cells
- Voltimeter readings in galvanic cells are called the standard cell voltage, E°cell, or the electromotive force, emf, or F
- A thermodynamically favored reaction will give a positive voltage reading. If a reaction is not thermodynamically favored, the reaction can be reversed to make it favorable
Standard Reduction Potentials
- E°cell = E°cathode - E°anode
- E°cathode is standard reduction potential for the reaction occurring at the cathode and represent tendency to remove electrons from the electrode surface
- E°anode is standard reduction potential for the reaction occurring at the anode and represent tendency to remove electrons from the anode
Standard Cell Voltage and Equilibrium
- E°cell = (.0591 / n)(log Keq)
- n is total number electrons transferred in redox reactions
- combining anode and cathode quotient get equilibrium constant, Keq
Free Energy Change, ΔG°, and Standard Cell Voltages
- ΔG° = -nF(E°cell)
- F is faraday’s constant
Important Redox Reactions
Combustion Reaction
- When organic compounds react with oxygen can cause combustion which produced carbon dioxide and water
- C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
Oxidation of Metals
- Magnesium burns bright white in oxygen. Often in fireworks
- Steel wool burns in a flame
- Aluminum is considered highly combustible. However, aluminum oxide formed produces impervious coating so complete oxidation does not occur
- Iron and steel react poorly. Rust requires water to occur.