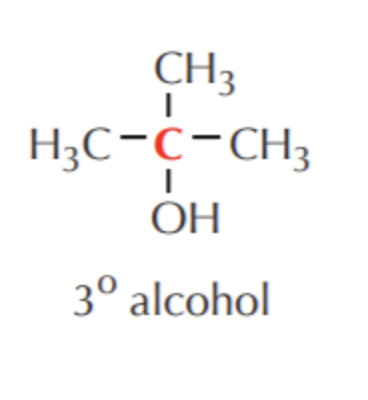Chem 1C OCHEM
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
ring
add Cyclo before the alkane
double bonded carbon-carbon bond
Alkene add -ene ending
Triple bonded carbon-carbon bond
Alkyne add -yne ending
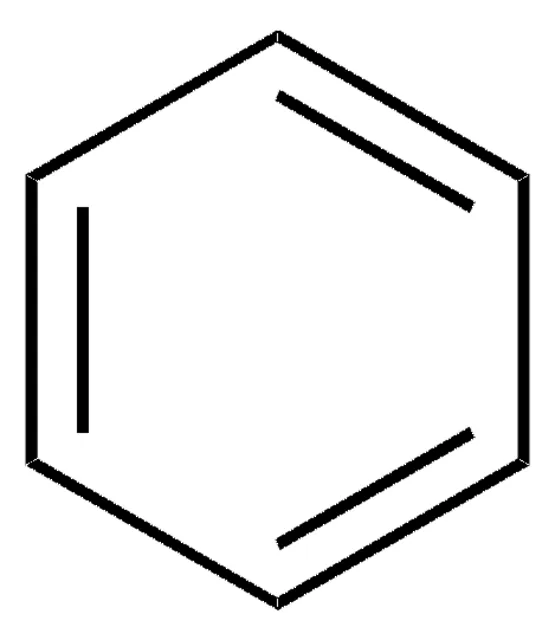
delocalized pi electrons throughout the ring
benzene
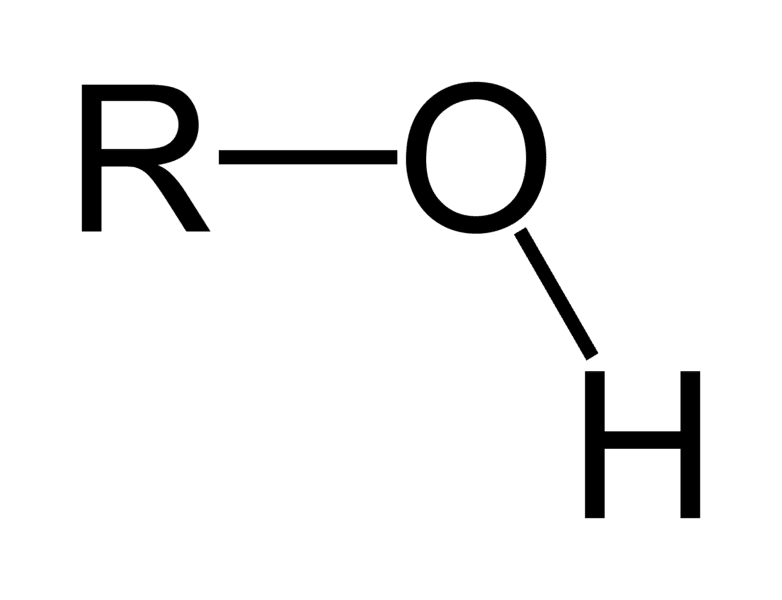
R-OH (OH at the end of a functional group)
alcohol, replace -e with -ol

O double bonded to carbon at the end of a chain
Aldehyde, replace -e with -al
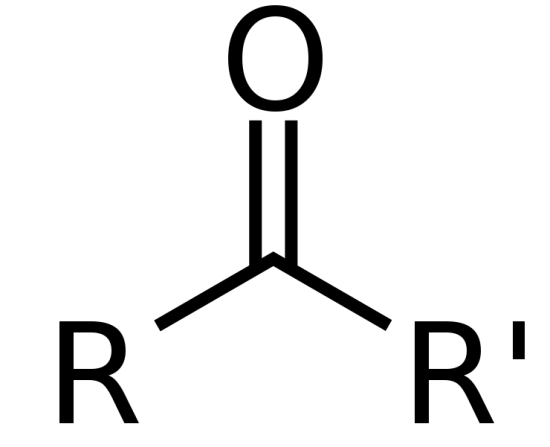
O doubled to carbon in-between two R groups
Ketone, replace -e with -one
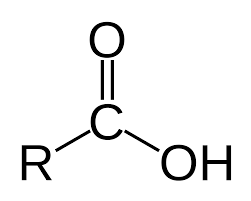
O double bonded to carbon with carbon being bonded to one OH and one R group.
Carboxylic acid, replace -e with -oic acid
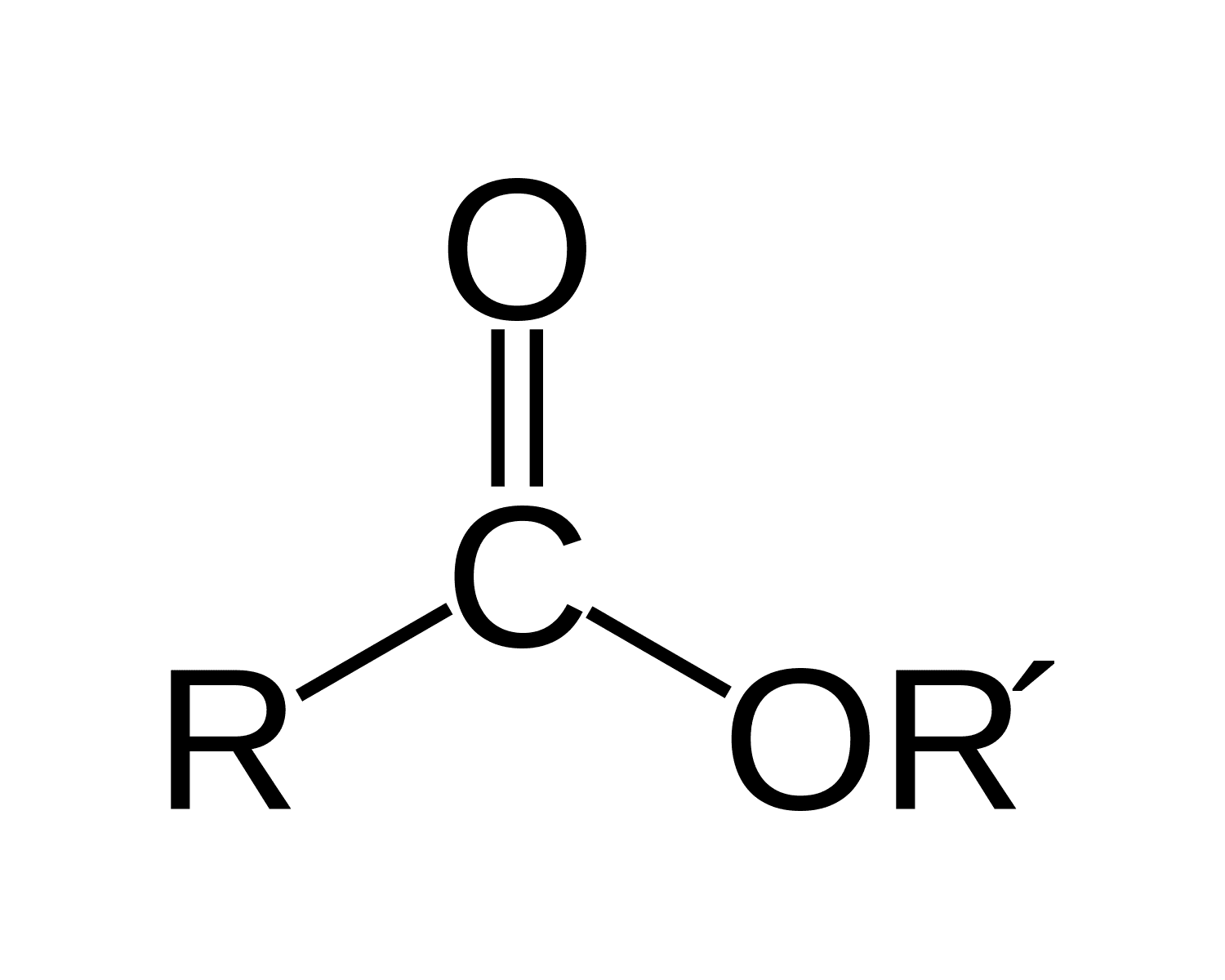
Ester : replace the ending of the carboxylic acid portion with: -oate, and the alchohol portion with -yl

Amine: replace ending with -Amine
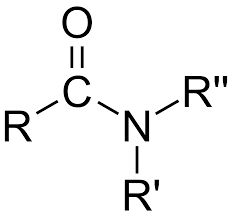
Amide: Replace ending with -amide

Substituent name _______
Isopropyl
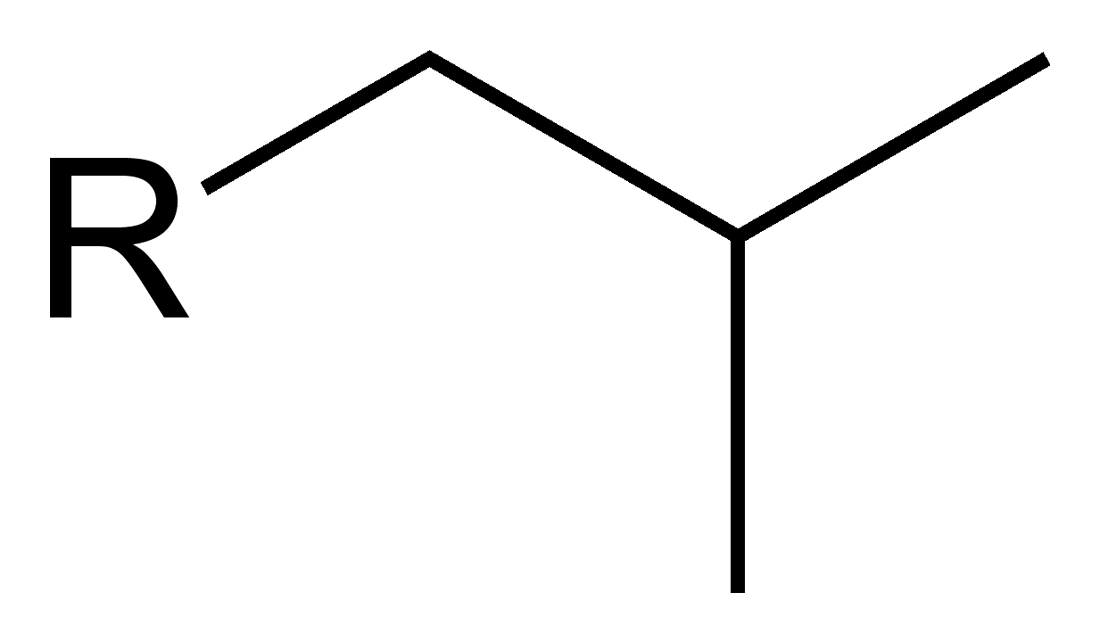
Substituent name _______
Isobutyl
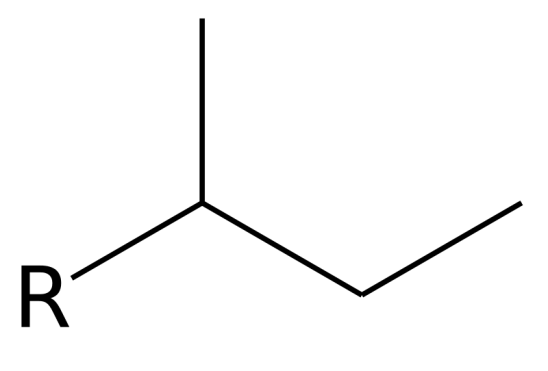
Substituent name _______
Sec-Butyl
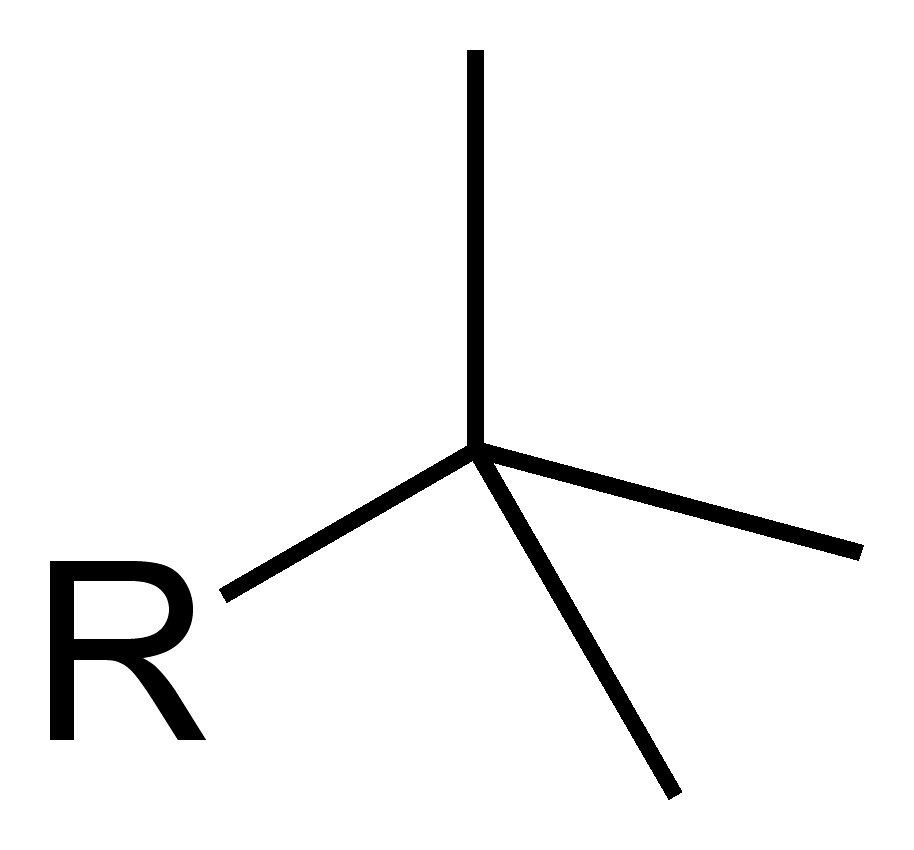
Substituent name _________:
Tert-Butyl
primary structure of protein
held together by covalent peptide/amide bonds
Secondary Structure
alpha helix or B pleated sheets, H-bonding
Tertiary structure
overall 3D shape, covalent bonds(disulfide) and IMFS
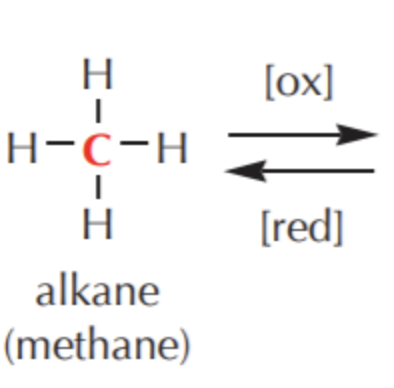
What do you get if you oxidize this?
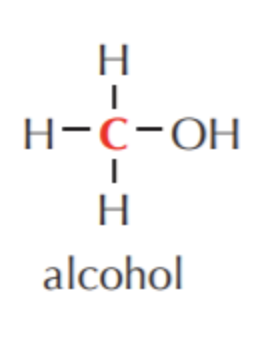
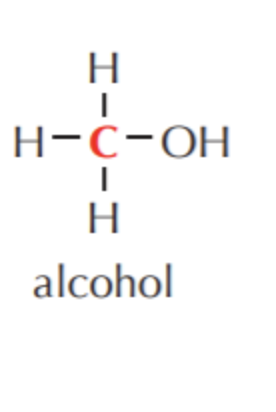
What do you get if you oxidize this?
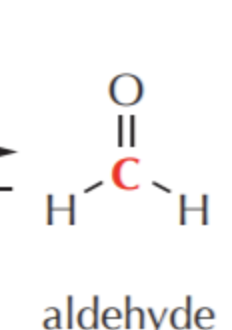
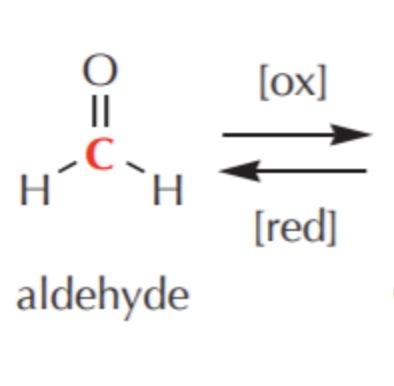
What do you get if you oxidize this?
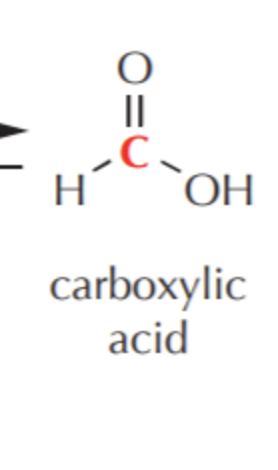
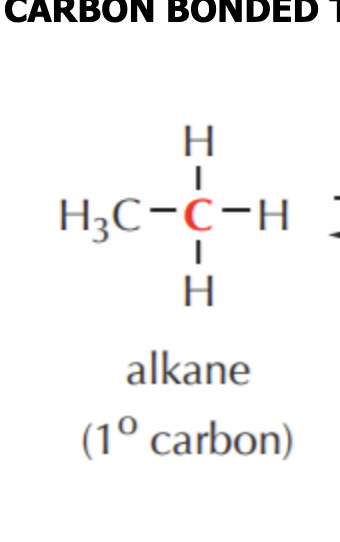
What do you get if you oxidize this?
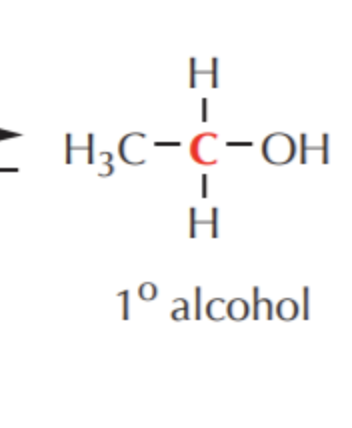
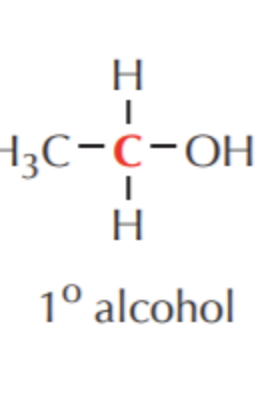
What do you get if you oxidize this?
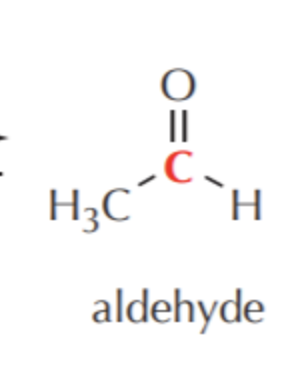
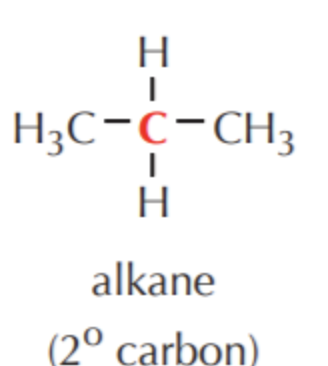
What do you get if you oxidize this?
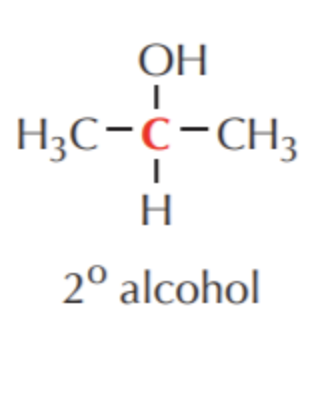
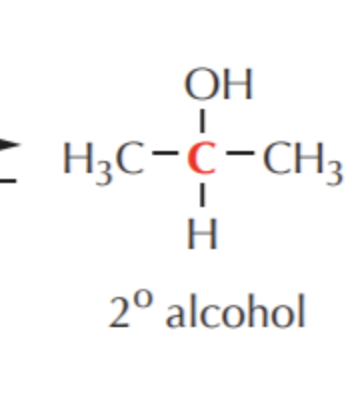
What do you get if you oxidize this?
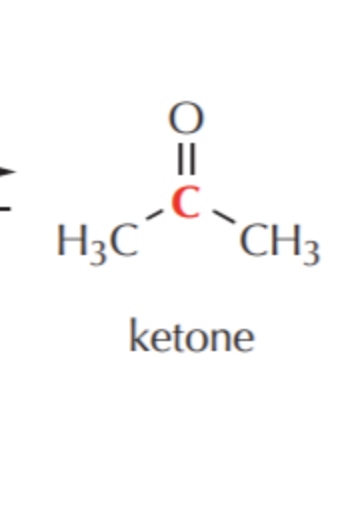
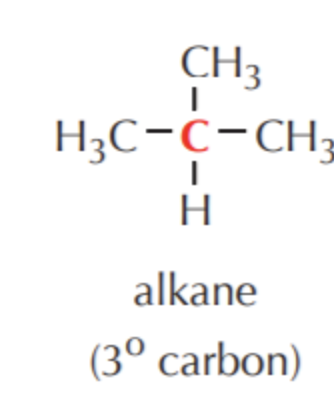
What do you get if you oxidize this?