BIO-110: Foundation of Cells: Atoms & (Macro)Molecules
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Matter
Not made or destroyed; it just gets redistributed in our universe
Abiogenesis
The life from nonlife
The elements for life
CHON(PS) (Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus, Sulfur)
Molecules
Multiple elements bonded together with different types of bonds
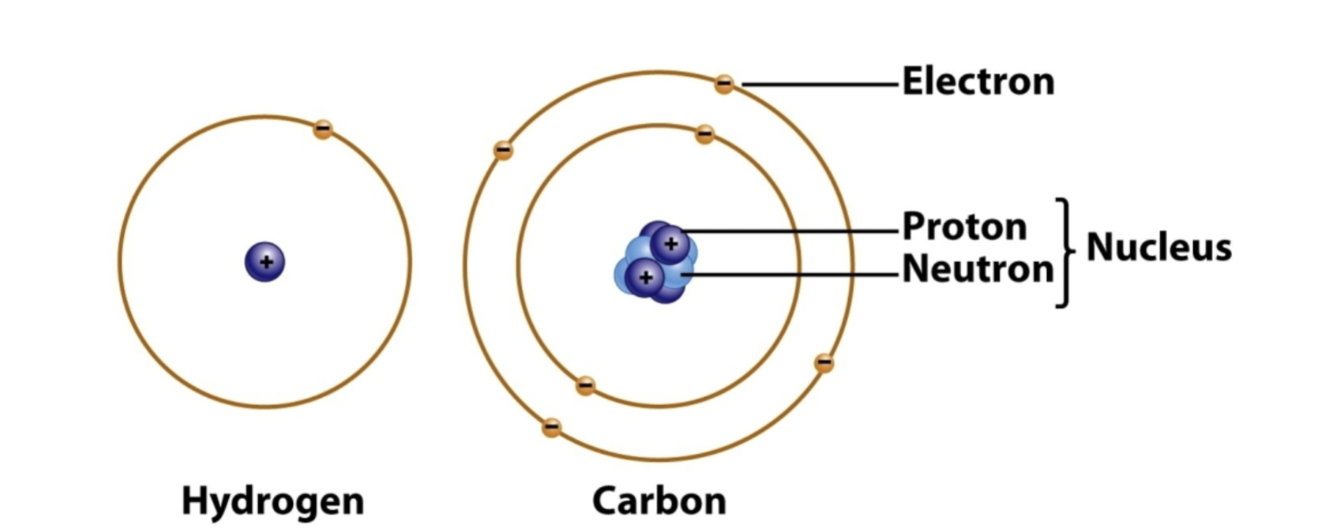
Atoms
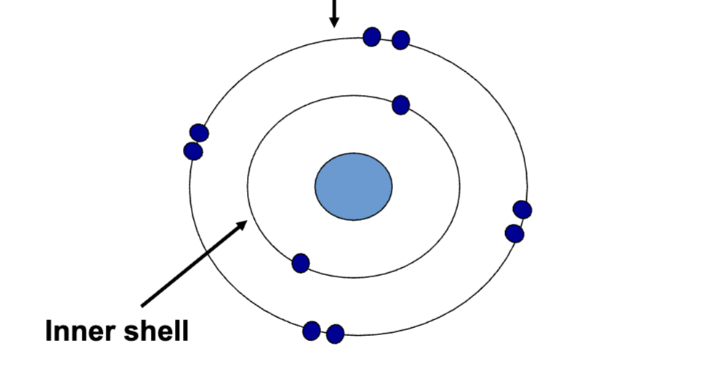
Valence shell
Only two can be paired, if there is a third, then it has to move to another shell
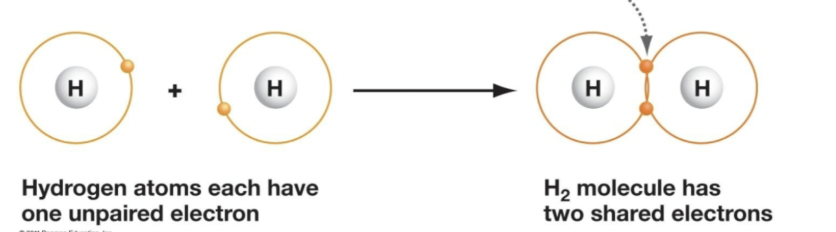
Covalent bonds
Formed when atoms share pairs of electrons; the sharing of electrons between one element and another
Electronegativity
Denotes how strongly an atom will attract electrons to itself
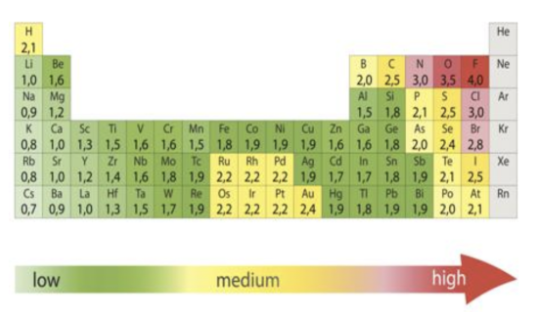
Ionic bonds
When one atom completely donates an electron to another
1st and 2nd law of Thermodynamics
Energy is not created or destroyed and entropy increases (in an isolated environment)
Chemical functional groups
Very common motifs that are found in the structure of molecules
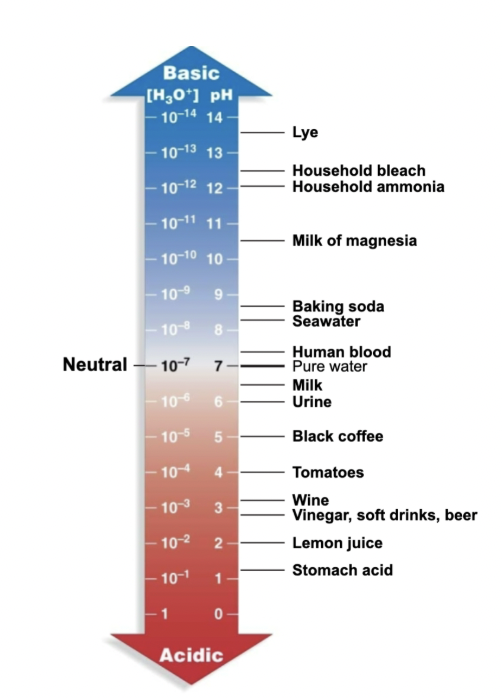
Acidity
Determined by the concentration of free hydrogen ions (protons) in a solution