collision theory
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
collision theory
in for particles to react when they collide, they must have sufficient energy and collide in correct orientation
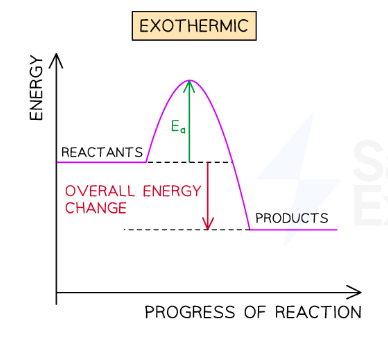
exothermic pathway
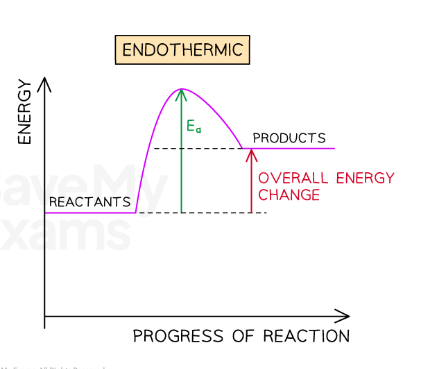
endothermic pathway
ineffective collisions
particles collide without sufficient kinetic energy to react…the reactive parts of the molecules may not be close enough to each other
effective collisions
collisions of particles leading to bond breaking and a chemical reaction
activation energy
minimum energy needed for colliding particles to successfully collide and cause a chemical reaction
increasing the reaction rate
collision frequency increases…proportion of colliding particles with energy higher than activation energy increases
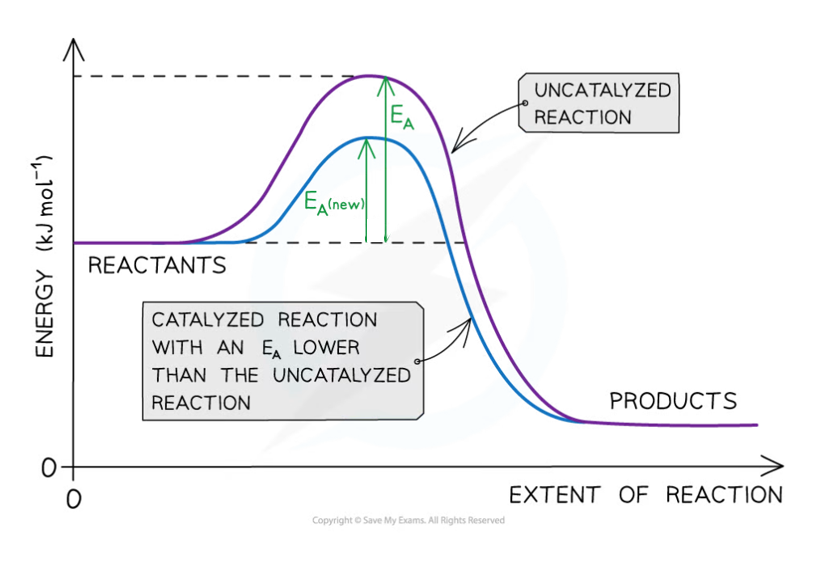
catalysts
substance increasing the rate of reaction by lowering activation energy but is unchanged at the end of the reaction
increasing concentration
increased frequency of collisions…the higher the concentration the more reacting particles available per unit volume
increasing pressure
increased frequency of gas molecule collisions
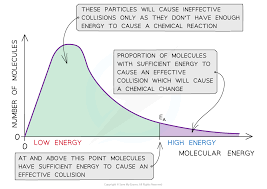
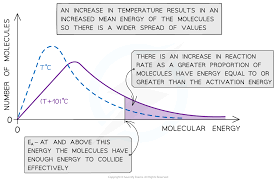
Boltzmann distribution
graph showing number of molecules with particular kinetic energy…shows that only few molecules have more energy than activation energy
increasing temperature
particles move around more quickly which increases frequency of collisions…proportion of successful collisions increases due proportion of molecules exceeding activation energy increases
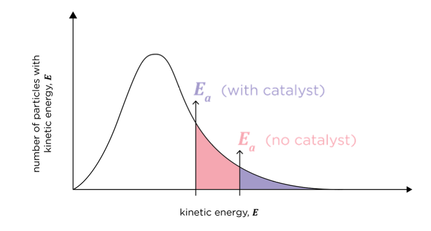
presence of catalysts on Boltzmann distribution
lower activation energy results in a greater proportion of molecules in the mixture having sufficient energy to react
homogenous catalysts
catalyst and reactants in a reaction are in the same phase
heterogenous
catalyst and reactions are in different phases of the reaction