PC4 1.2.1 Only molecules
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
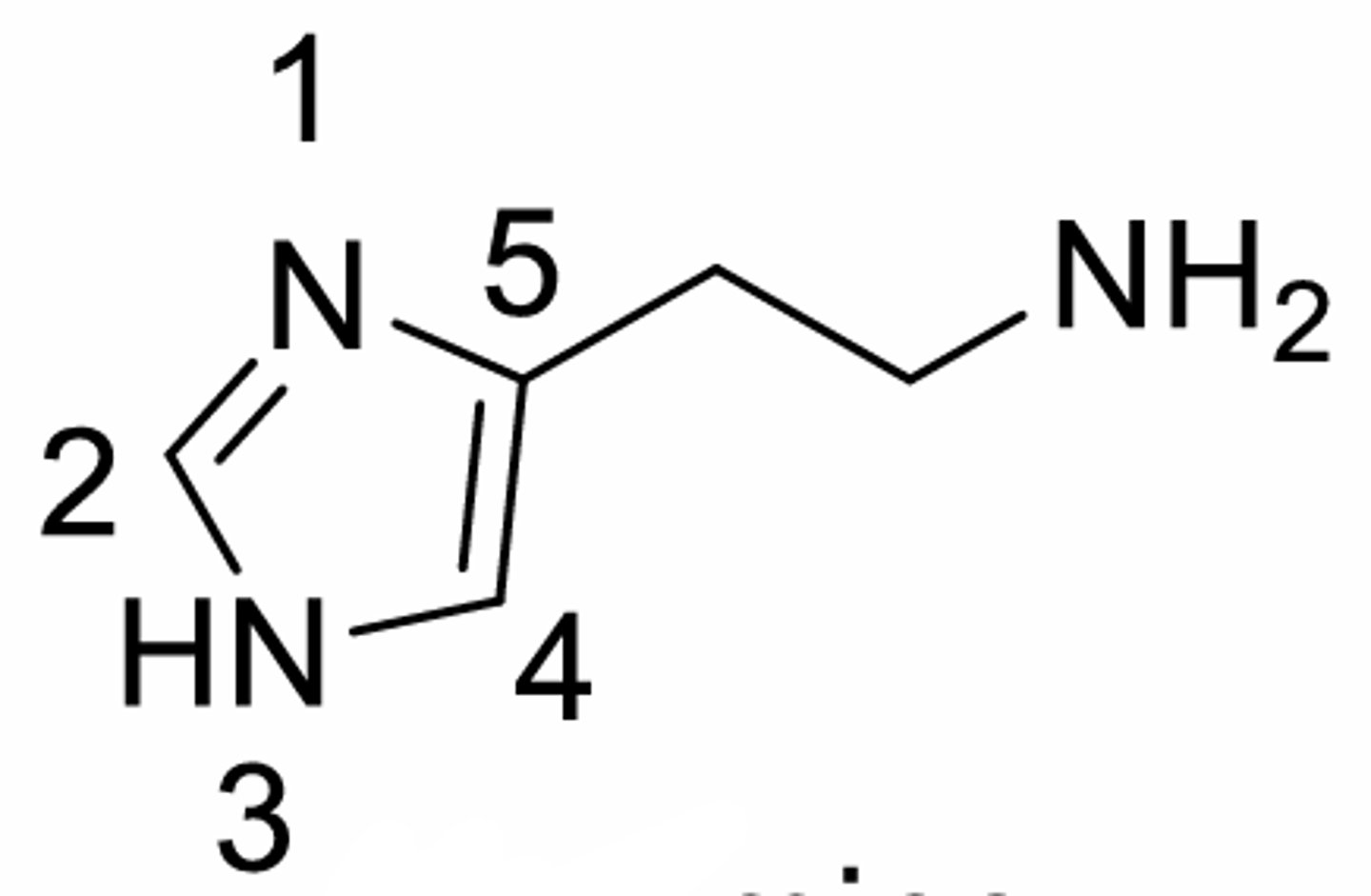
Histamine
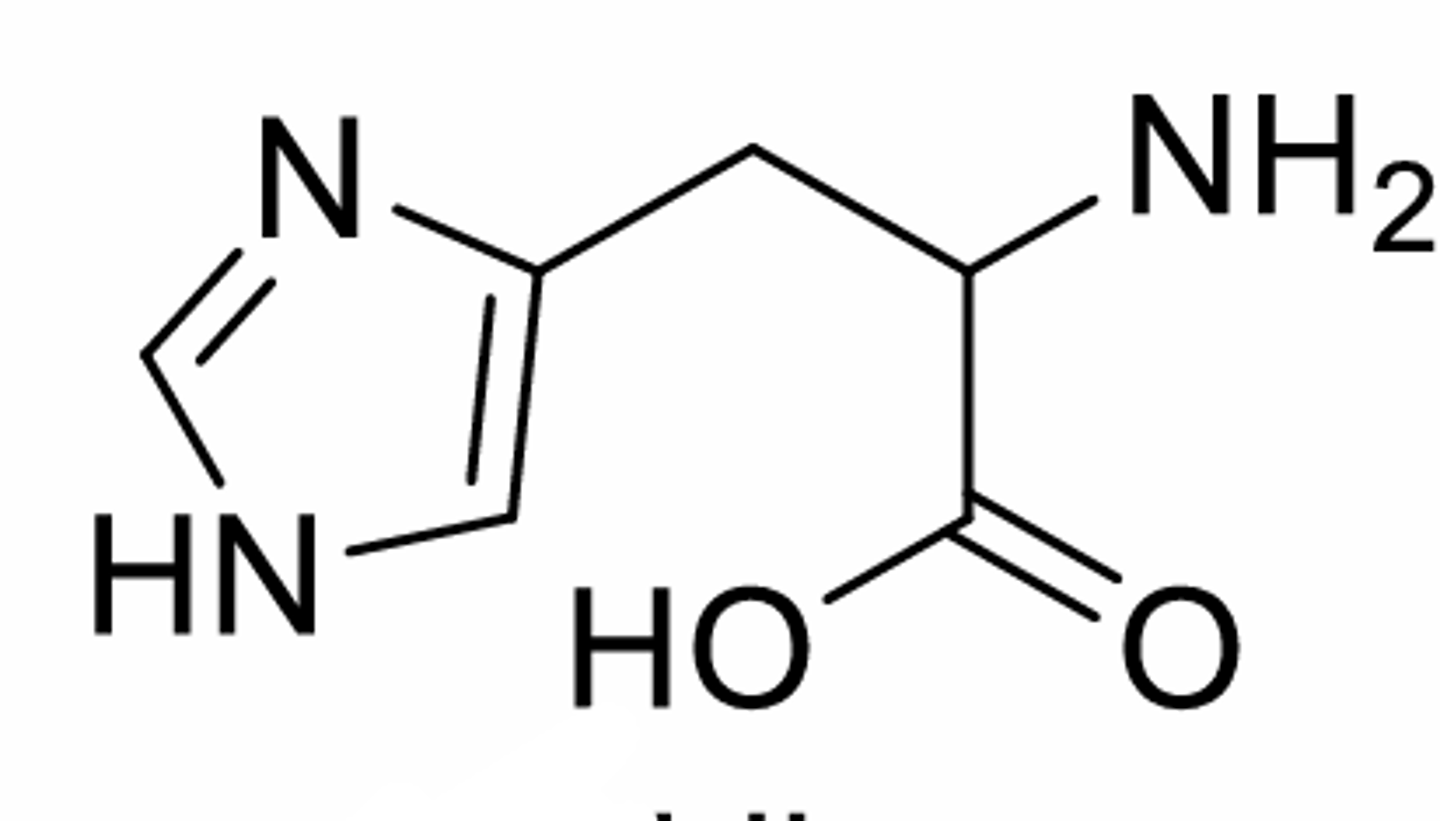
Histidine
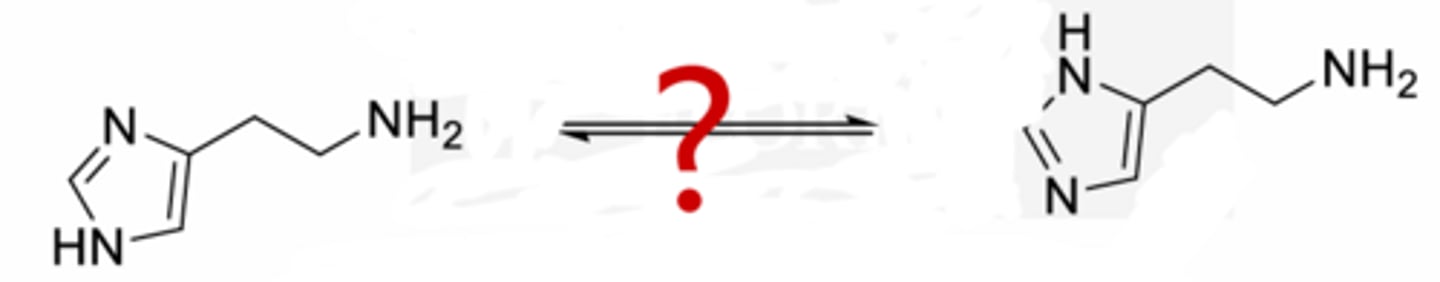
Tautomers of histamine
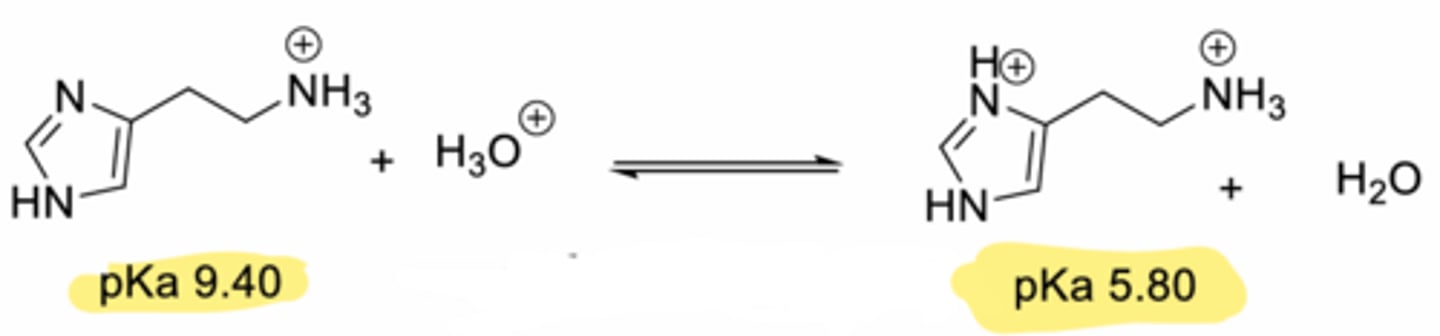
Protonated species of histamine (active form)
[Monocation makes up >96%]
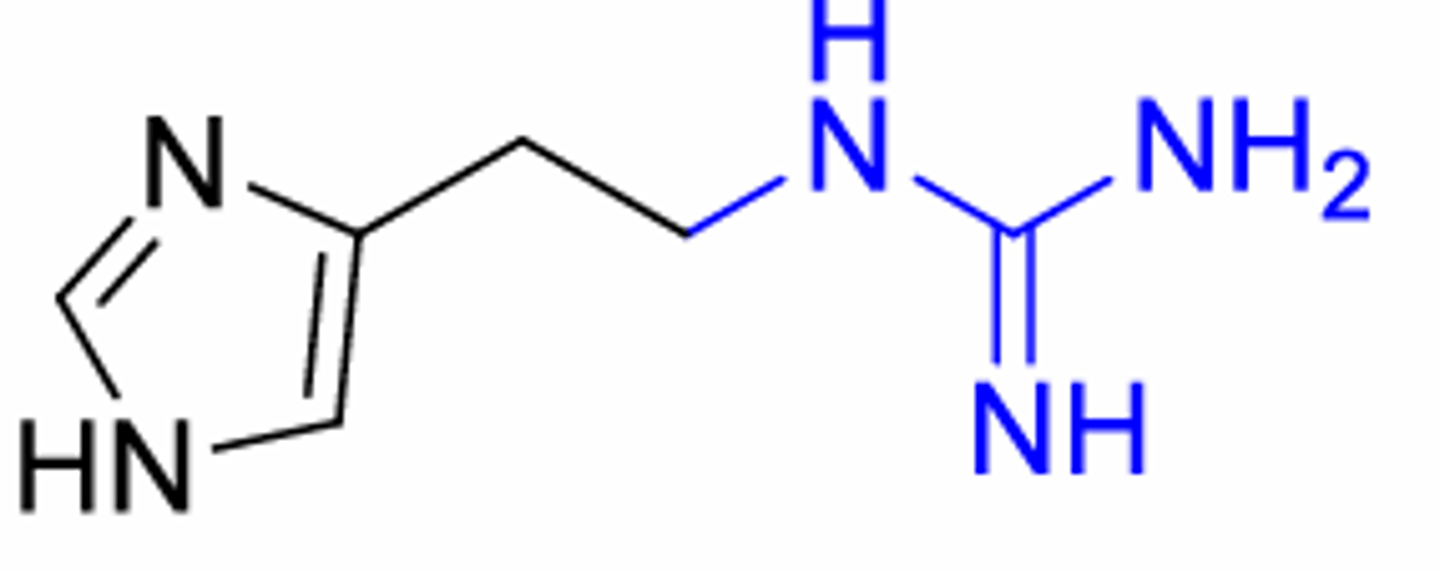
What did blue group lead to
Discovery of H2 antagonists: Introducing a neutral functional group (blue) leads to an antagonist
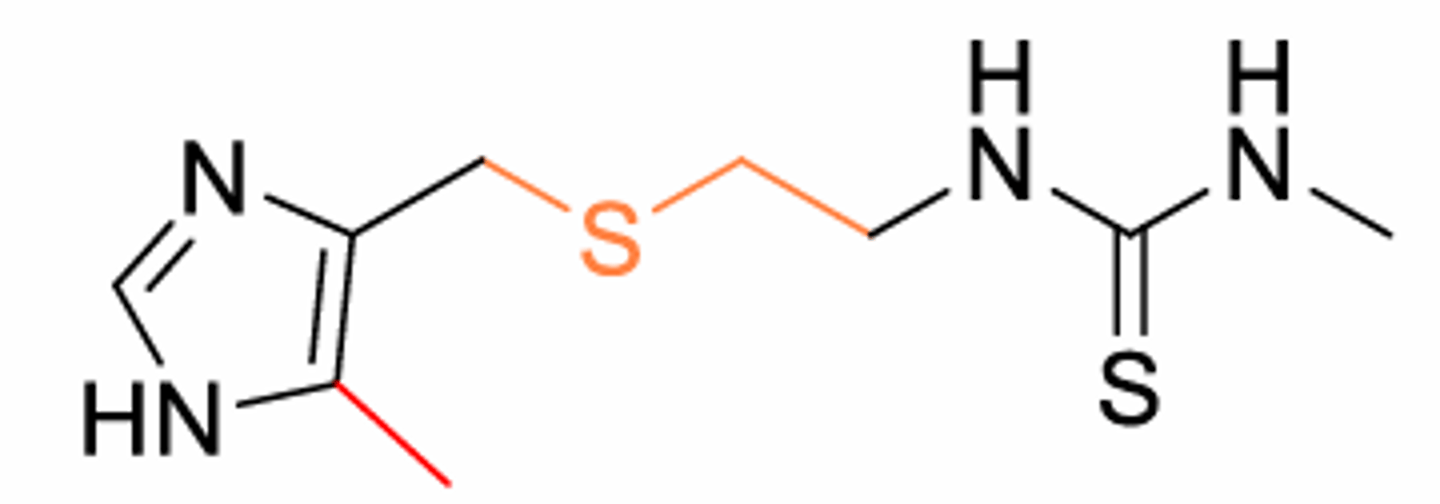
What did orange part lead to?
Discovery of H2 antagonists: Extend the linker (orange) increases the potency of the antagonist
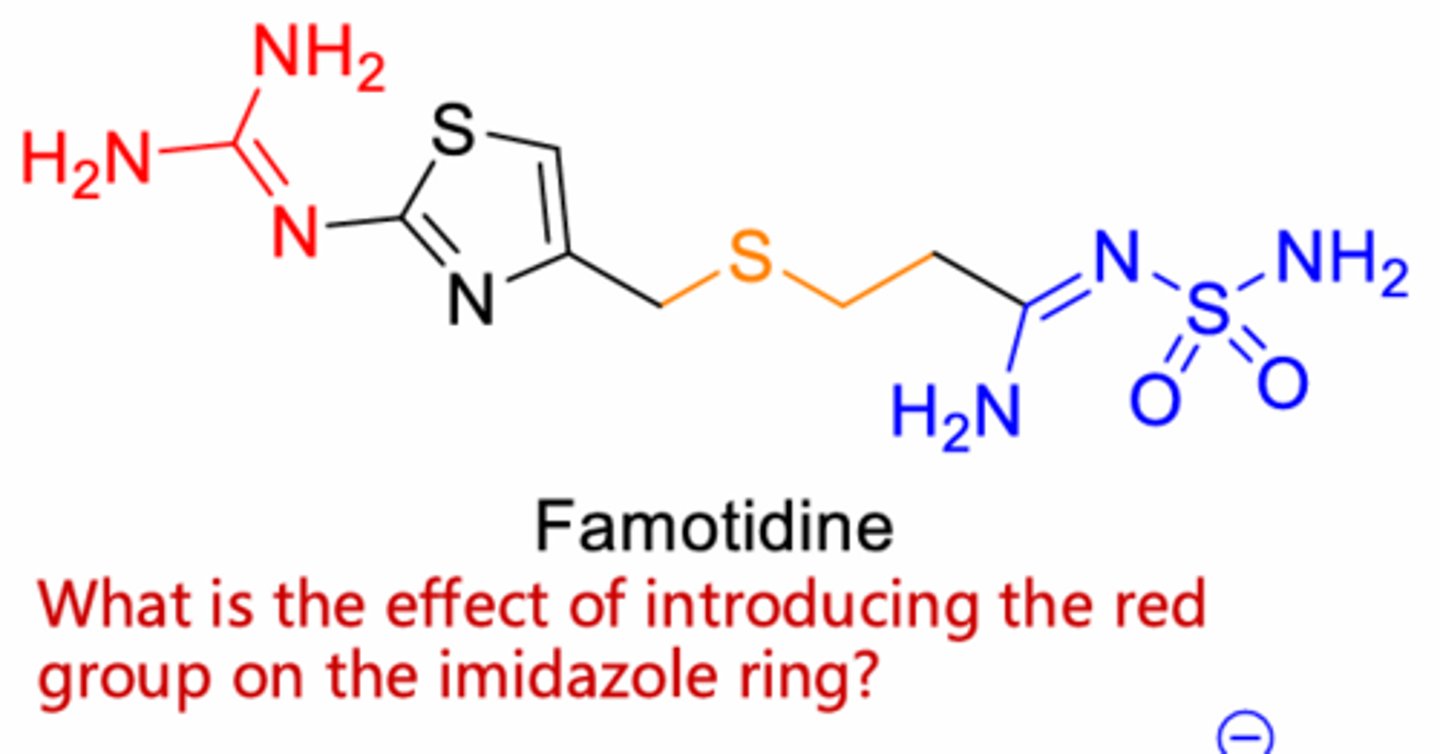
Increase H2 selectivity
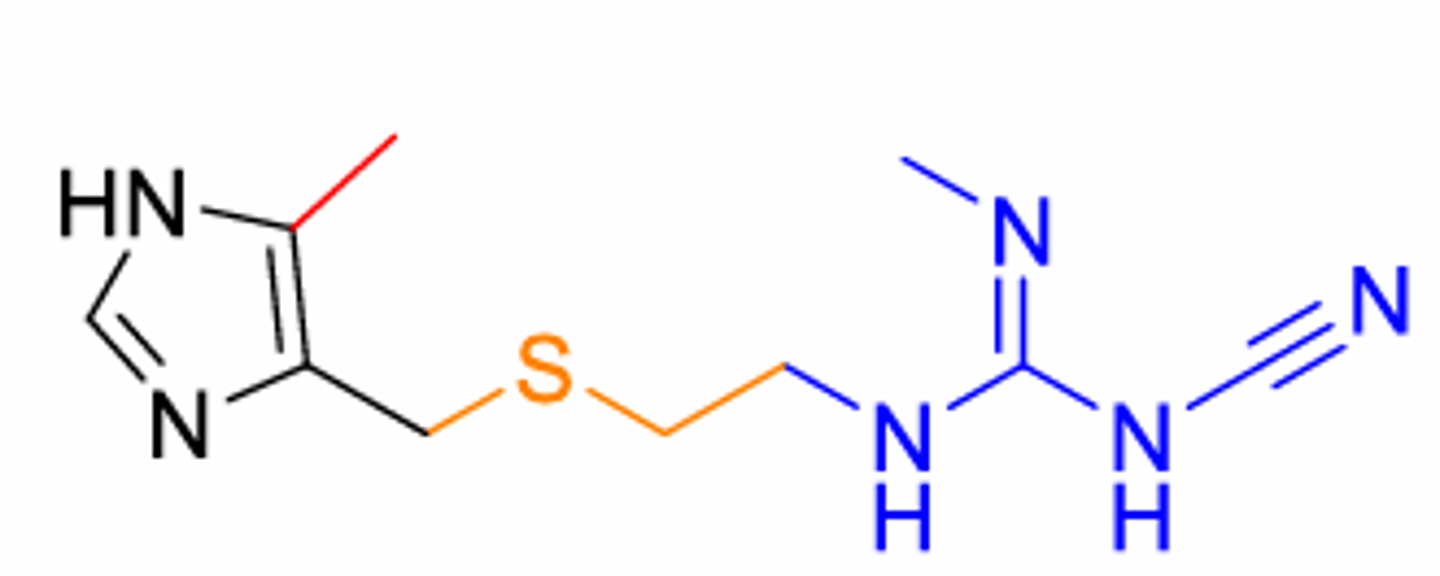
What is this structure
Cimetidine (1st H2 antagonist). Still has imidazole
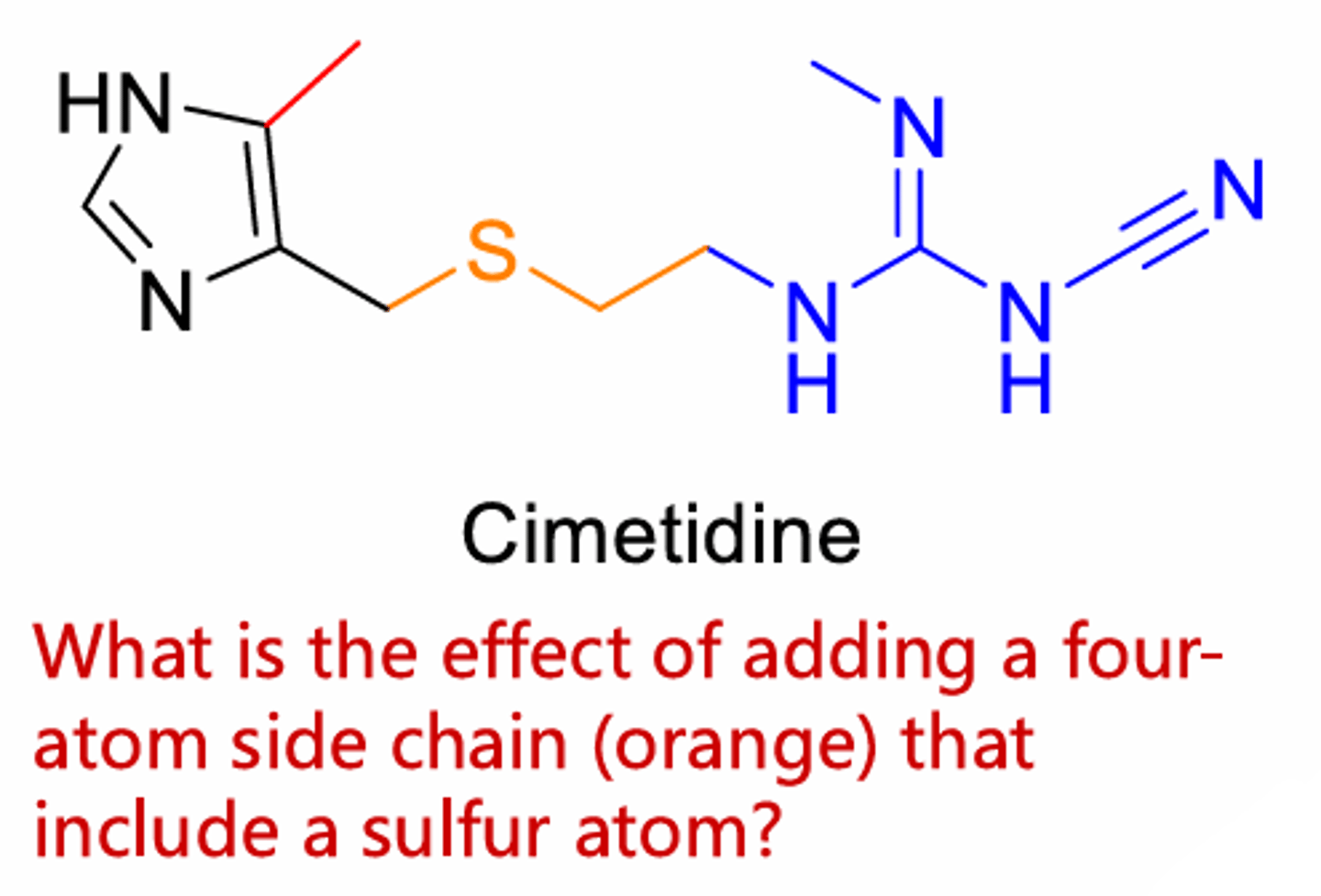
The sulfur atom increases potency compared to carbon or oxygen
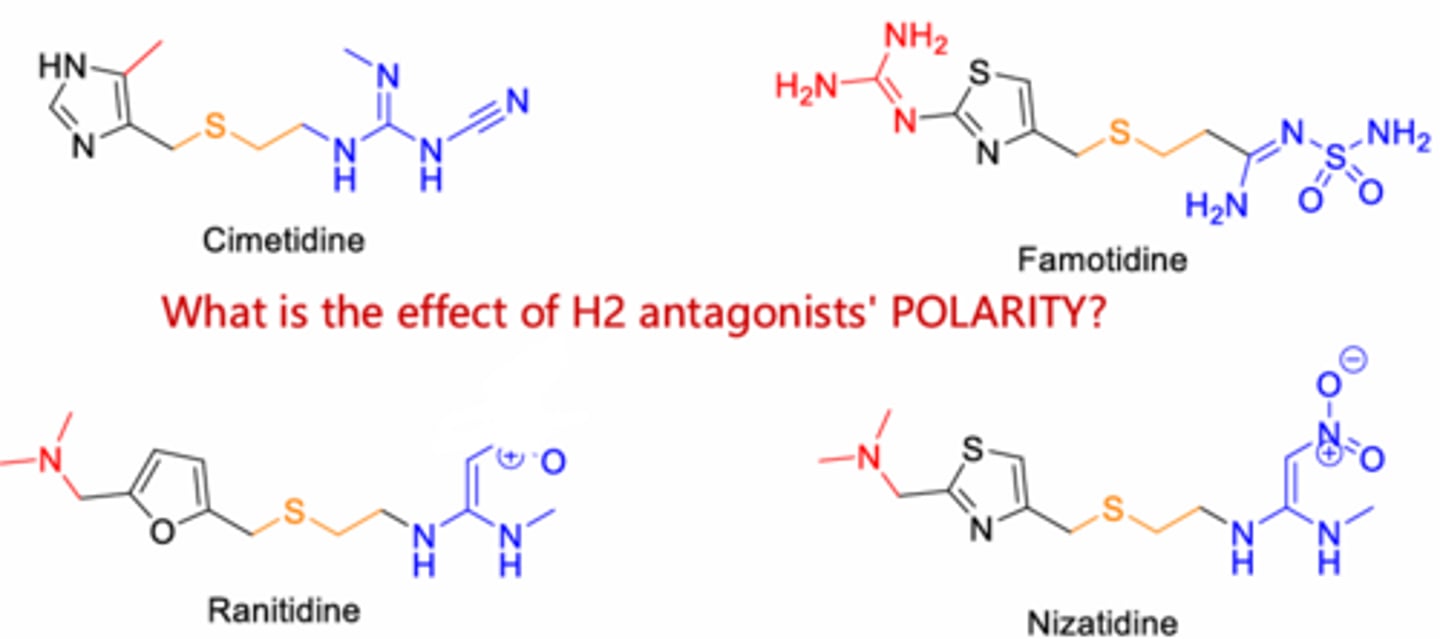
More available in the periphery and to a lesser extent in the CNS
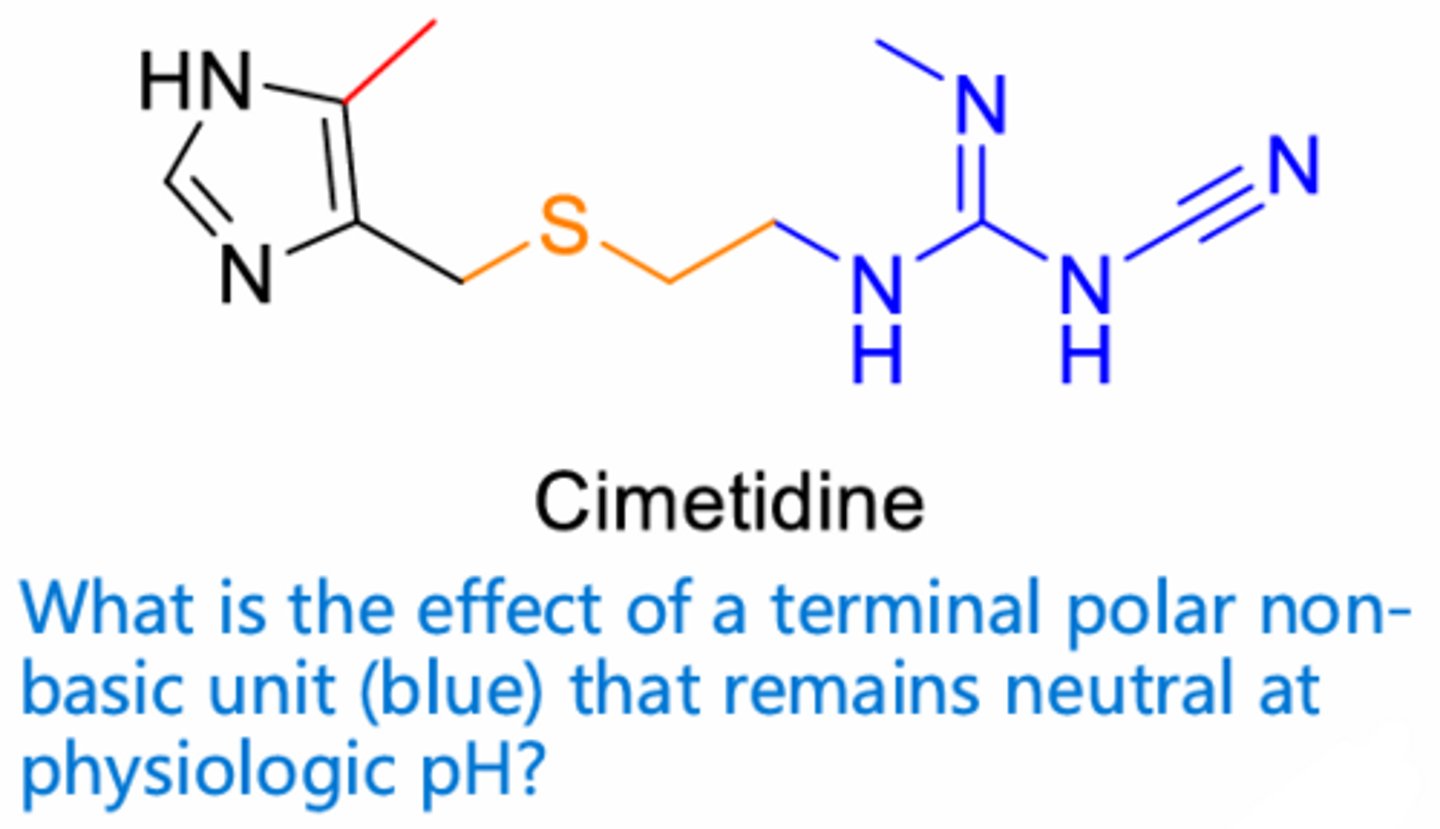
ensure antagonism without receptor activation
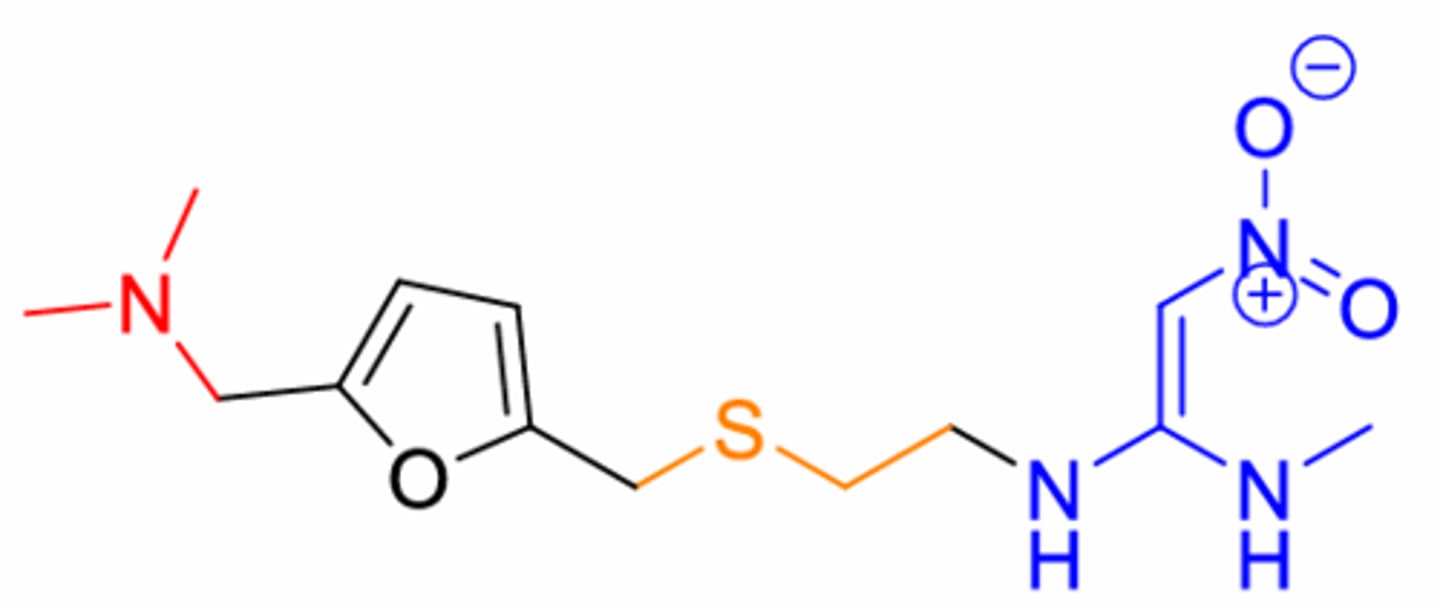
What is this structure? O furan ring with double O ketone one end
Ranitidine
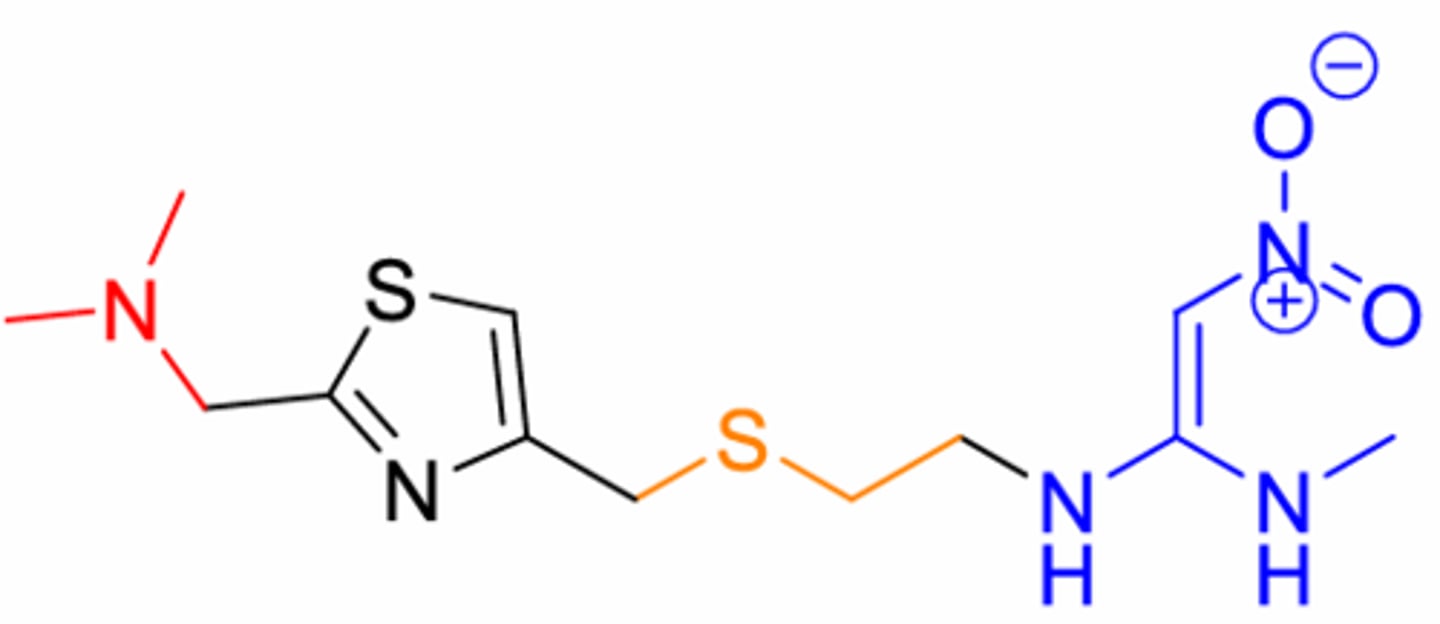
What is this structure. Sulfur ring plus double O ketone on end
Nizatidine
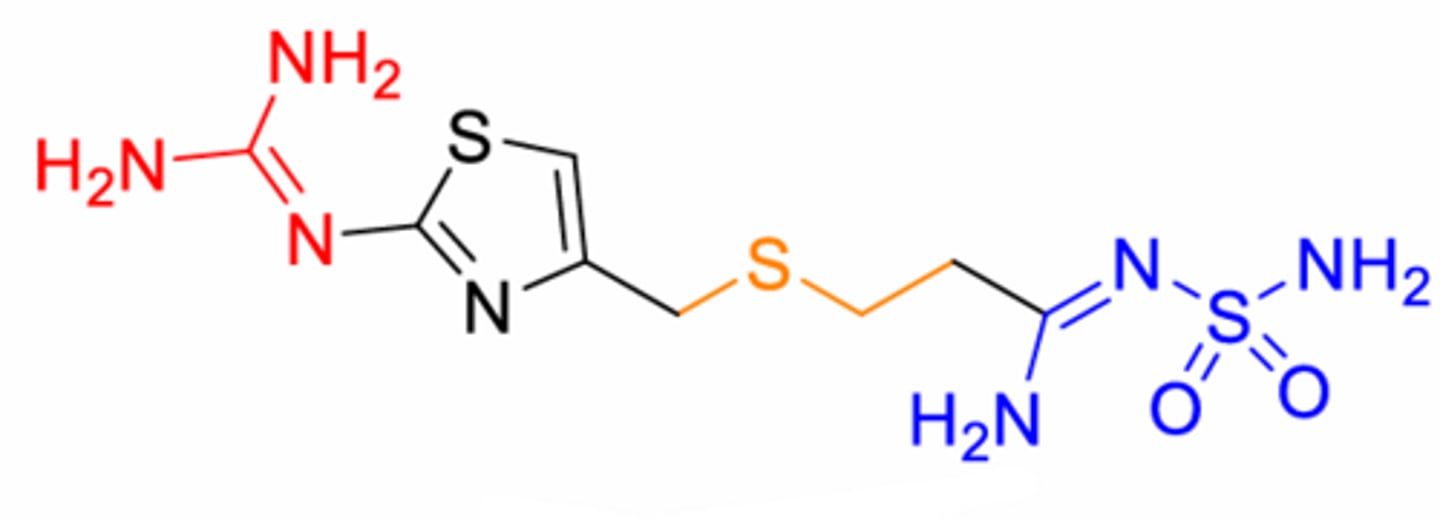
What is this structure? Double sulfur structures
Famotidine
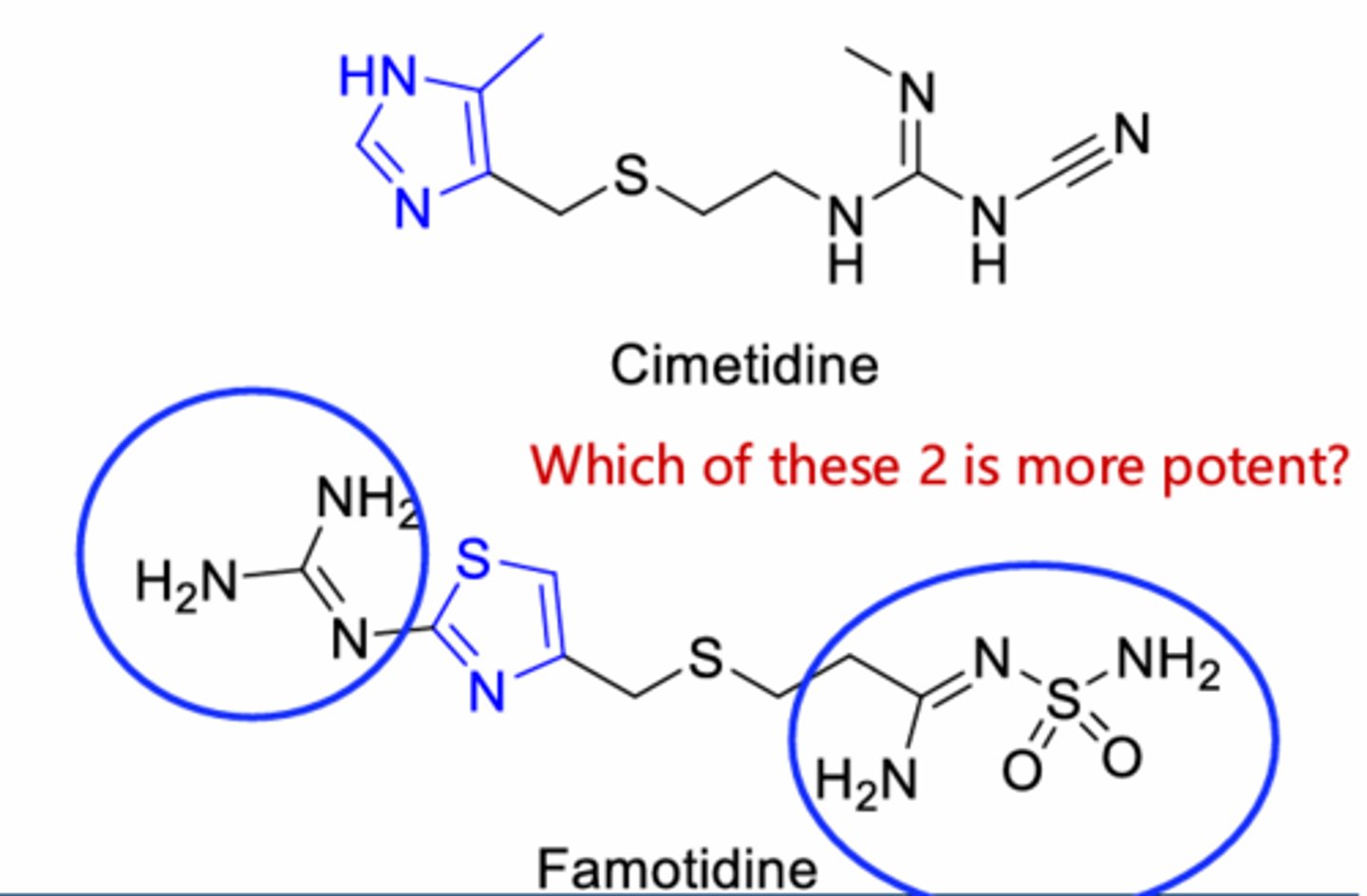
Famotidine > Cimetidine
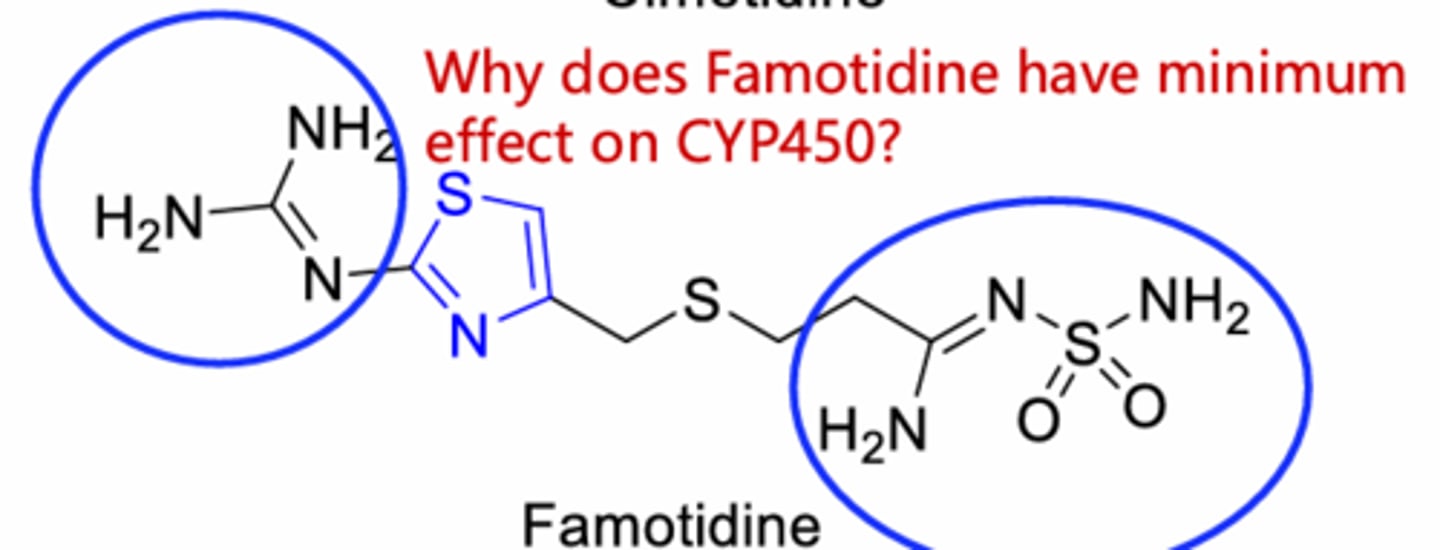
lack of imidazole functional group
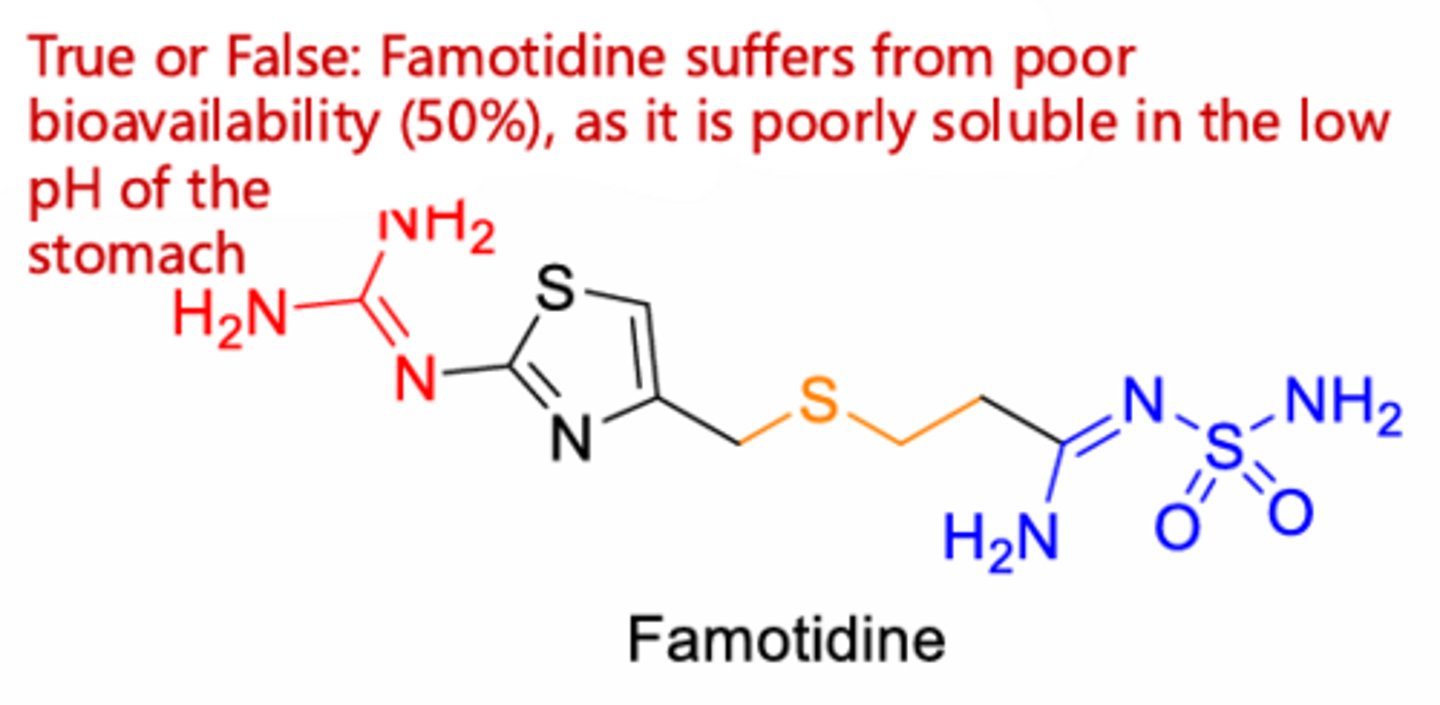
True
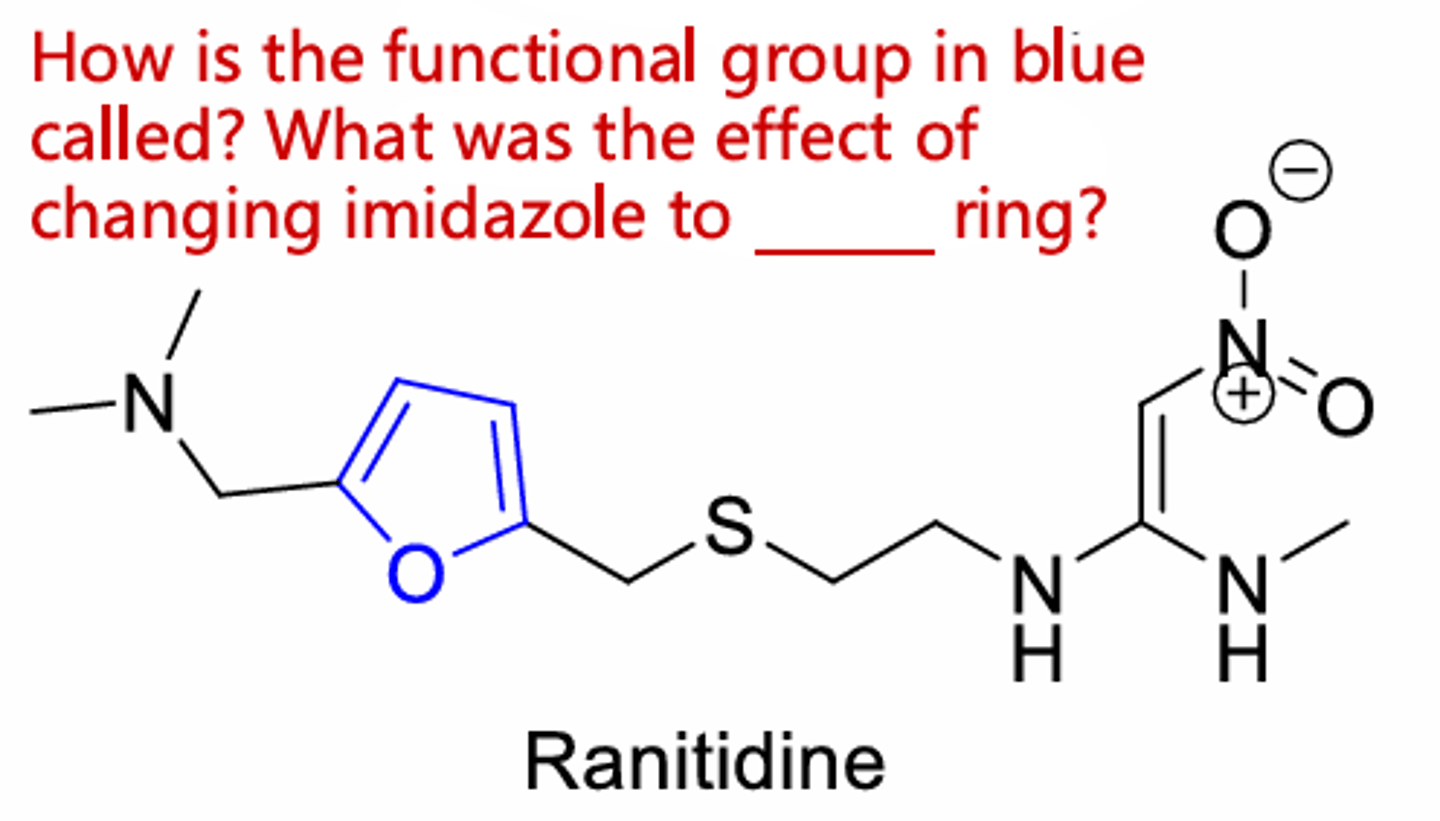
Furan ring, improves tolerability (less side effects)
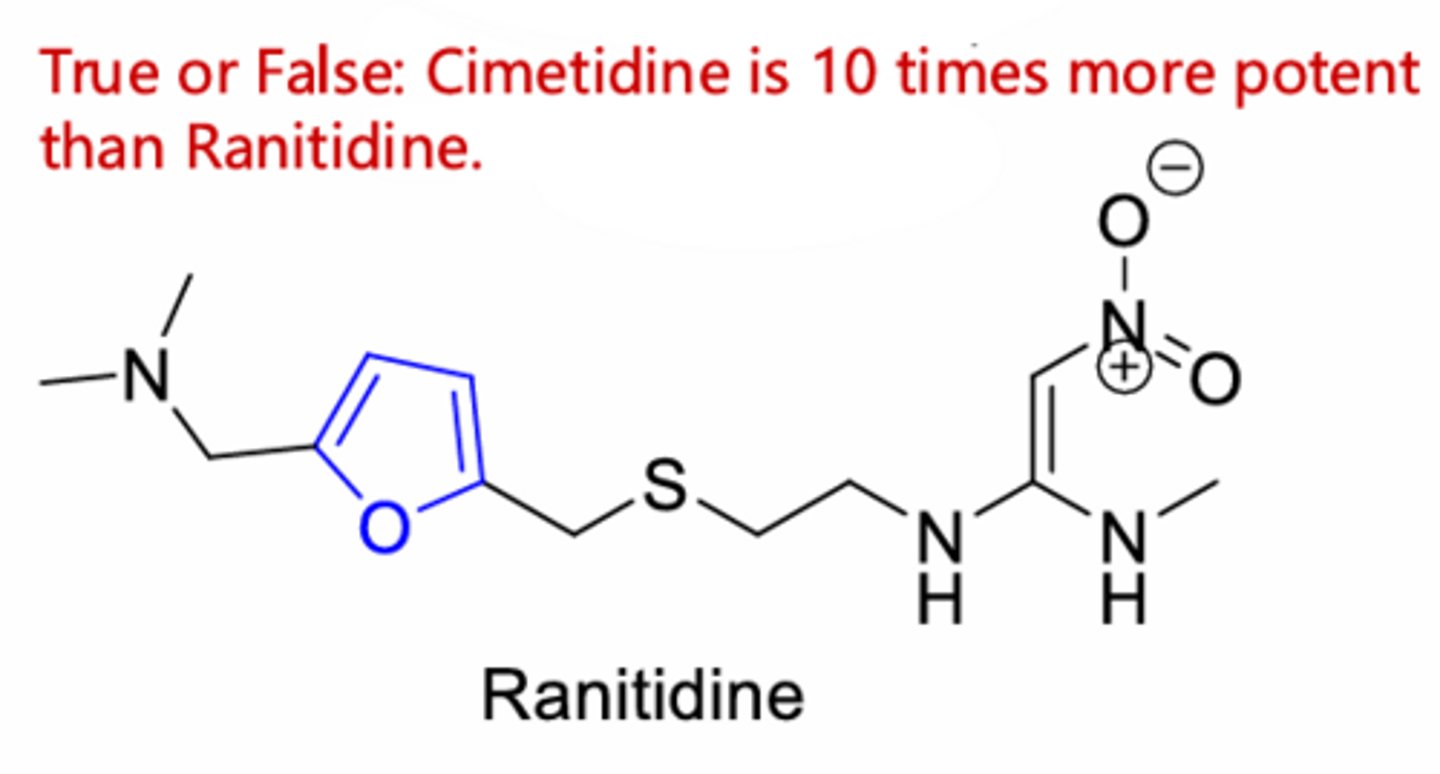
False, ranitidine is 10 times more potent
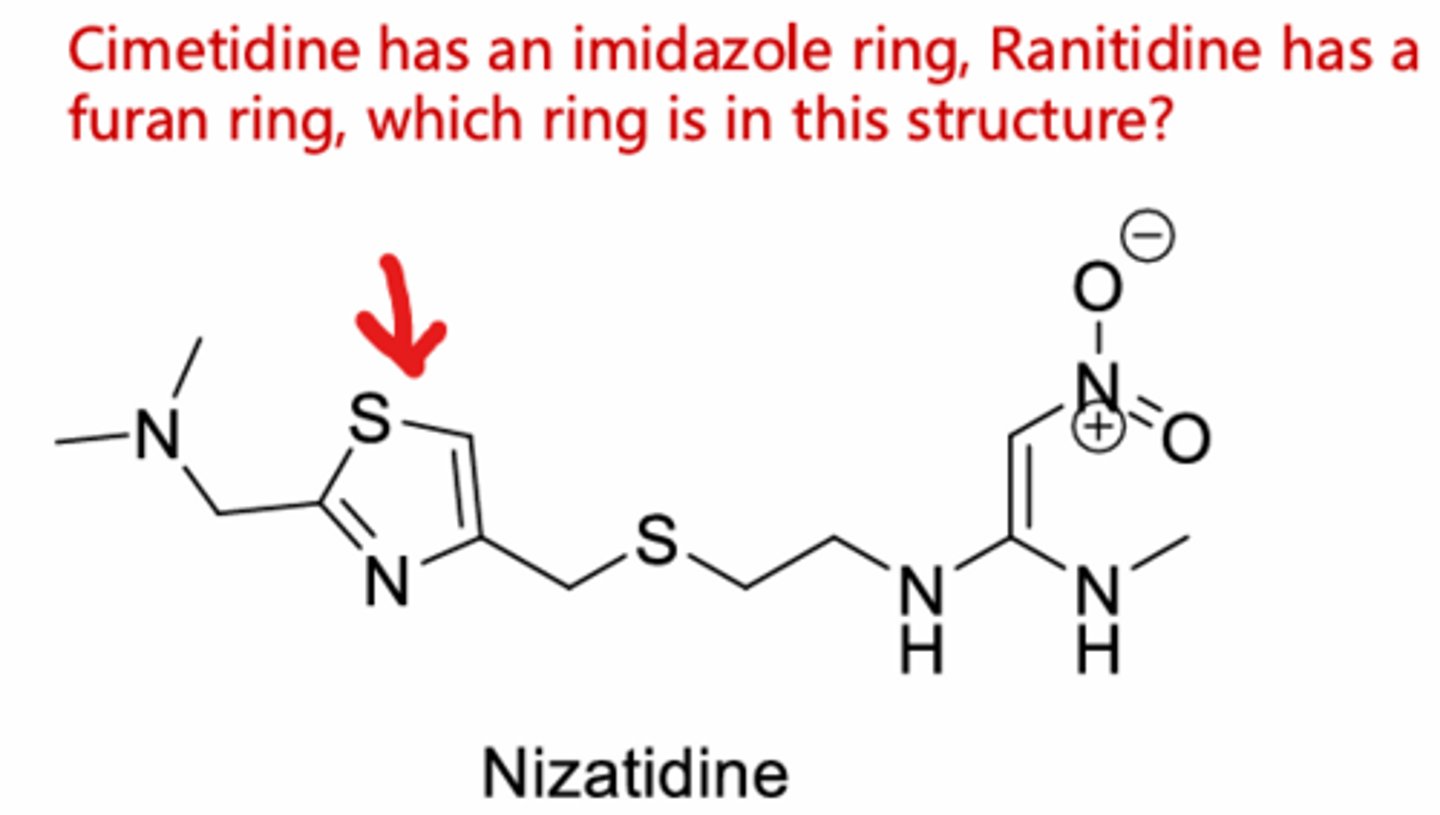
Thiazole
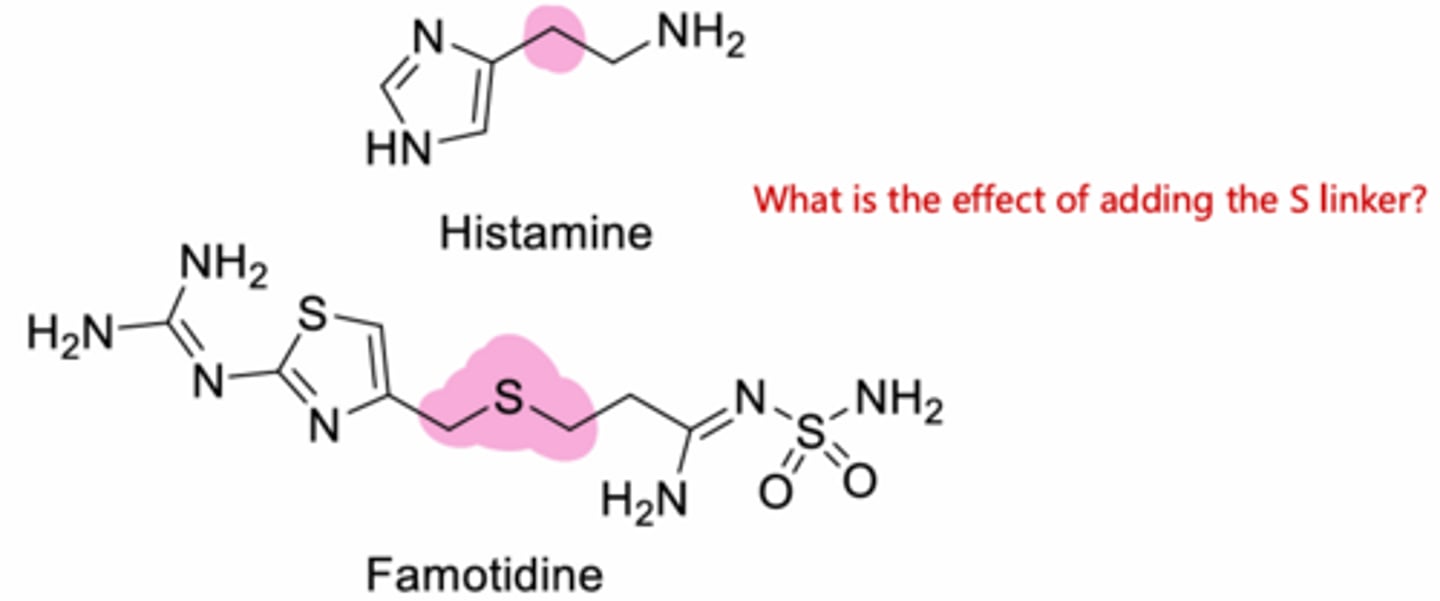
Increases the potency