PRINCIPLES OF STAINING
0.0(0)
Card Sorting
1/132
There's no tags or description
Looks like no tags are added yet.
Last updated 6:21 PM on 4/16/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
133 Terms
1
New cards
STAINING
● Process of applying dyes on the sections to see and study the architectural pattern of the tissue and physical characteristics of the cells.
2
New cards
Acidic structures
are attracted to basic dyes
3
New cards
Basic structures
are attracted to acidic dyes
4
New cards
METHODS OF STAINING
1\. Direct staining 2. Indirect staining 3. Progressive staining 4. Regressive staining
5
New cards
Hematoxylin
stains the nuclear stain
6
New cards
Eosin
stains the cytoplasmic structure
7
New cards
DIRECT STAINING
Process of giving color to the sections using aqueous / alcoholic dye solutions
Example: Methylene blue and eosin
Example: Methylene blue and eosin
8
New cards
INDIRECT STAINING
● Action of dye is intensified by adding another agent, mordant, or accentuator. These will intensify the action of the dye
9
New cards
Mordant
● serves as a link or a bridge between the tissue and the dye to make the tissue staining possible
● there are times that the dye alone may only stain weakly or none at all
● The mordant combines with the dye to form a colored lake. In turn, it combines with the tissues to form a tissue-mordant dye complex that is rendered insoluble in ordinary aqueous or alcoholic solvents
● This allows subsequent counter staining and dehydration to be carried out easily. It is an integral part of the staining reaction. Without which, no staining could possibly occur
● A mordant may be applied to the tissue before the stain or it may be included as part of the staining technique or it may be added to the dye solution itself.
Examples:
\- Potassium alum with hematoxylin in
\- Ehrlich’s hematoxylin Iron in Weigert’s hematoxylin
More examples: ● Aluminum ● Iron ● Phosphotungstic Acid
● Lead ● Copper ● Molybdenum
● there are times that the dye alone may only stain weakly or none at all
● The mordant combines with the dye to form a colored lake. In turn, it combines with the tissues to form a tissue-mordant dye complex that is rendered insoluble in ordinary aqueous or alcoholic solvents
● This allows subsequent counter staining and dehydration to be carried out easily. It is an integral part of the staining reaction. Without which, no staining could possibly occur
● A mordant may be applied to the tissue before the stain or it may be included as part of the staining technique or it may be added to the dye solution itself.
Examples:
\- Potassium alum with hematoxylin in
\- Ehrlich’s hematoxylin Iron in Weigert’s hematoxylin
More examples: ● Aluminum ● Iron ● Phosphotungstic Acid
● Lead ● Copper ● Molybdenum
10
New cards
Accentuator
● It speeds up the staining reaction by increasing the staining power and selectivity of the dye. ● Not essential to the chemical union of the tissue and the dye because it does not participate in the staining reaction. ● Only accelerates or hastens the speed of the staining reaction by increasing the staining power and selectivity of the dye
Examples
* Potassium hydroxide in Loeffler’s methylene blue
* Phenol in Carbol thionine and carbolfuschin
Examples
* Potassium hydroxide in Loeffler’s methylene blue
* Phenol in Carbol thionine and carbolfuschin
11
New cards
PROGRESSIVE STAINING
● Tissue elements are stained in a definite sequence ● Staining solution is applied for specific periods of time or until the desired intensity of coloring of the different tissue elements is attained ● Less favored than regressive staining due to difficulty of producing sufficiently intense progressive staining of cells structures without staining other parts ● Once the dye is taken up by the tissue, it is not washed nor decolorized. Thereby, resulting in diffuse color and obscure details
12
New cards
REGRESSIVE STAINING
● The tissue is first overstained to obliterate the cellular details ● Excess stain is removed or decolorized from unwanted parts of the tissue, until the desired intensity of color is obtained
13
New cards
DIFFERENTIATION
● The selective removal of excess stain from the tissue during regressive staining BSM1 ● Usually done by: washing in simple solution; use of acids and oxidizing agents
● Example: Alcohol - acts as a differentiator for both basic and acidic dyes
● If the primary stain used is a basic dye the differentiating agent is carried out by an acid solution
● Example: Alcohol - acts as a differentiator for both basic and acidic dyes
● If the primary stain used is a basic dye the differentiating agent is carried out by an acid solution
14
New cards
METACHROMATIC STAINING
● Uses specific dyes which differentiate particular substances by staining them with a color different from that of the stain itself
● Employed for: cartilage, connective tissues, epithelial mucins, mast cell granules, and amyloid
● preferred staining for tissues in frozen sections
● Metachromatic tissues consists of acid mucopolysaccharides:
1\. Cartilage
2\. Connective tissue mucin
● Employed for: cartilage, connective tissues, epithelial mucins, mast cell granules, and amyloid
● preferred staining for tissues in frozen sections
● Metachromatic tissues consists of acid mucopolysaccharides:
1\. Cartilage
2\. Connective tissue mucin
15
New cards
Cartilage
chondroitin sulfate
16
New cards
Connective tissue mucin
acid mucopolysaccharides\`
17
New cards
All metachromatic dyes
aere cations/basic whose peculiar staining property depends upon their tendency to polymerize
18
New cards
All tissue components showing metachromasia
are large anionic (negative) or acidic molecules containing large amounts of sulfate, phosphate or carboxylic acid radicals.
19
New cards
COUNTER STAINING
● The application of a different color or stain to provide contrast and background to the staining of the structural components to be demonstrated ● It will stain the parts that were not stained by the primary stain.
20
New cards
Cytoplasmic stains
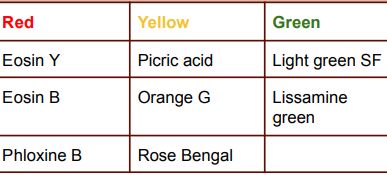
21
New cards
Nuclear stains
Red - Neutral red , Safranin O. Carmine, hematoxylin
Blue - Methylene, Toluidine, Celestine
Blue - Methylene, Toluidine, Celestine
22
New cards
METALLIC IMPREGNATION
● Employs a colorless solution of metallic salts for specific tissue elements, which are reduced by the tissue, producing an opaque, usually black deposit on the surface of the tissue or bacteria
● Examples: ○ Ammoniacal silver ○ Gold chloride ○ Silver nitrate ■ Avoid using metallic instruments
● A metallic impregnating agent is different from a stain in that it is not absorbed by the tissue, but is held physically on the surface as a precipitate or as a reduction product in certain tissue components
● Examples: ○ Ammoniacal silver ○ Gold chloride ○ Silver nitrate ■ Avoid using metallic instruments
● A metallic impregnating agent is different from a stain in that it is not absorbed by the tissue, but is held physically on the surface as a precipitate or as a reduction product in certain tissue components
23
New cards
IMPREGNATION
● makes use of heavy metal salts ● utilized for silver staining of the nervous system ● used to demonstrate reticulin
● Metal salts selectively precipitate on certain cellular and tissue components
● Metal salts selectively precipitate on certain cellular and tissue components
24
New cards
Silver nitrate
can be used as impregnating & staining agent
25
New cards
VITAL STAINING
● The selective staining of living cell constituents
● Examples:
○ Cytoplasmic staining to visualize phagocytosis
○ True vital staining where mitochondria is demonstrated
● The nucleus of a living cell is resistant to vital stains and therefore cannot be demonstrated. In fact, demonstration of nuclear structures during vital staining suggests permeability of the membrane of the dye which signifies the death of the cell.
● Examples:
○ Cytoplasmic staining to visualize phagocytosis
○ True vital staining where mitochondria is demonstrated
● The nucleus of a living cell is resistant to vital stains and therefore cannot be demonstrated. In fact, demonstration of nuclear structures during vital staining suggests permeability of the membrane of the dye which signifies the death of the cell.
26
New cards
INTRAVITAL STAINING
● Done by injecting the dye into any part of the animal body, producing specific coloration of certain cells
● Common dyes: lithium, carmine, India ink
● Common dyes: lithium, carmine, India ink
27
New cards
SUPRAVITAL STAINING
● Used to stain living cells immediately after removal from the living body
● This is what we use for staining reticulocytes or polychromatophilic erythrocytes, Heinz bodies, and hemoglobin H
**Common dyes used**:
1\. Neutral red: best vital dye
2\. Janus green: demonstrates mitochondria
3\. Trypan blue: 1 hr standing will cause toxicity to the cell
4\. Nile blue
5\. Thionine
6\. Toluidine blue
● This is what we use for staining reticulocytes or polychromatophilic erythrocytes, Heinz bodies, and hemoglobin H
**Common dyes used**:
1\. Neutral red: best vital dye
2\. Janus green: demonstrates mitochondria
3\. Trypan blue: 1 hr standing will cause toxicity to the cell
4\. Nile blue
5\. Thionine
6\. Toluidine blue
28
New cards
STAINING OF PARAFFIN SECTIONS
● we have to drain to ensure moisture has evaporated ● If there is incomplete drying, section may detach from the slide
● After the section is cut and mounted on the slide, it must be drained and dried thoroughly to ensure moisture has evaporated. ● Xylene used must be subsequently removed with absolute alcohol followed by descending grades of alcohol to prevent damage and detachment of tissues
**Steps**:
1\. First wash of xylene to remove paraffin
2\. Tissue sections are subjected to decreasing alcohol concentrations
3\. Tissue sections are subjected to increasing alcohol concentrations
4\. Lastly, wash with xylene to increase refractive index of the glass slide
● If an alcohol stain is to be used, there is no more need to replace the alcohol in water. After deparaffinization with xylene, the section is subjected to decreasing grades of alcohol, in term: SECTIONS TO ALCOHOL
● After the section is cut and mounted on the slide, it must be drained and dried thoroughly to ensure moisture has evaporated. ● Xylene used must be subsequently removed with absolute alcohol followed by descending grades of alcohol to prevent damage and detachment of tissues
**Steps**:
1\. First wash of xylene to remove paraffin
2\. Tissue sections are subjected to decreasing alcohol concentrations
3\. Tissue sections are subjected to increasing alcohol concentrations
4\. Lastly, wash with xylene to increase refractive index of the glass slide
● If an alcohol stain is to be used, there is no more need to replace the alcohol in water. After deparaffinization with xylene, the section is subjected to decreasing grades of alcohol, in term: SECTIONS TO ALCOHOL
29
New cards
Coplin jar
5-9 slides
30
New cards
Slotted staining dishes
5-19 slides
31
New cards
Metal or glass staining racks or carries
can carry 10-30 slides
32
New cards
CLASSIFICATION OF STAINS
1\. Histological staining
2\. Histochemical staining (Histochemistry)
3. Immunohistochemical staining
2\. Histochemical staining (Histochemistry)
3. Immunohistochemical staining
33
New cards
HISTOLOGICAL STAINING
● A process of direct interaction of the tissue with a dye or staining solution
● Examples
○ Micro-anatomic stains ○ Bacterial stains ○ Specific tissue stains (Muscles, Connective tissue and Neurologic)
■ Muscle and connective tissue are used to demonstrate the general relationships of tissues and cells with differentiation of nucleus and cytoplasm
● Examples
○ Micro-anatomic stains ○ Bacterial stains ○ Specific tissue stains (Muscles, Connective tissue and Neurologic)
■ Muscle and connective tissue are used to demonstrate the general relationships of tissues and cells with differentiation of nucleus and cytoplasm
34
New cards
Histochemical staining (Histochemistry)
● Tissues constituents are studied through chemical reactions that will permit microscopic localization of a specific tissue substance
● Examples:
○ Staining for the demonstration of hemoglobin crystals and carbohydrates for hemoglobin
○ Perl’s prussian blue reaction for hemoglobin
○ Periodic Acid Schiff for carbohydrates
● Active reagent used is the substrate
● Upon which the enzyme reacts and the final opacity or coloration produced is from the substrate rather than the tissue
● Examples:
○ Staining for the demonstration of hemoglobin crystals and carbohydrates for hemoglobin
○ Perl’s prussian blue reaction for hemoglobin
○ Periodic Acid Schiff for carbohydrates
● Active reagent used is the substrate
● Upon which the enzyme reacts and the final opacity or coloration produced is from the substrate rather than the tissue
35
New cards
Perl’s prussian blue
for hemoglobin
* Stains the storage form of iron (Fe3+) from the bone marrow aspirate to check for certain diseases like hemochromatosis.
Ingredients: Potassium ferrocyanide and hydrochloric acid→ blue precipitate
* Stains the storage form of iron (Fe3+) from the bone marrow aspirate to check for certain diseases like hemochromatosis.
Ingredients: Potassium ferrocyanide and hydrochloric acid→ blue precipitate
36
New cards
Periodic Acid Schiff
for carbohydrates
* Stains the glomerular basement membrane of the kidneys→ fuchsia pink
* Stains the glomerular basement membrane of the kidneys→ fuchsia pink
37
New cards
Turnbull’s Blue Reaction
→ Fe2+ (ferrous) form of iron
38
New cards
IMMUNOHISTOCHEMICAL STAINING
● Antibodies and antigens are used ● Immunologic + Histochemical techniques ● Allows phenotypic markers to be detected and demonstrated under the microscope ● Employs polyclonal/monoclonal, fluorescent labeled or enzyme-labeled antibodies ● Antibodies acts as signal molecules and they label the antigen
39
New cards
Polyclonal antibodies
composition of two or more cells or antibodies that have different genetic architecture/constitution
40
New cards
Monoclonal antibodies
uses hybridoma technology created by George Kohler and Cesar Milstein; makes use of a purified single celled-organism or antibodies; same genetic construction/arrangement
41
New cards
Fluorescent-labeled antibodies
antibodies that have fluorescent colors to easily identify them under the microscope because they will fluoresce
42
New cards
Enzyme-labeled antibodies
when adding a substrate the same as for enzyme histochemistry; the substrate will act upon the enzymes and tissue so the final coloration is produced by the substrate and not the tissue
43
New cards
H & E STAINING TECHNIQUE
● Most common method utilized for microanatomical studies ● Progressive or regressive staining may be used ● We only decolorize or differentiate when tissues are subjected to regressive staining
44
New cards
ORTHOCHROMATIC STAINING
● Tissues are stained in color shades that are similar to the color of the dye itself
45
New cards
BLUEING
● Alum hematoxylin stains nuclei with red color, which is converted to the familiar blue black when the section is washed in a weak alkali solution. ● Tap water is usually alkaline enough to produce this color change, but occasionally alkaline solutions such as saturated lithium carbonate, 0.5% ammonia in distilled water, or Scott's tap water substitute are necessary.
46
New cards
NEGATIVE STAINING
● Stains are not taken up by their tissue targets. ● The shapes and structures are disclosed by outlining or filling them with a stain (example in demonstrating the canaliculi of bone matrix using picro-thionine)
47
New cards
STAINING METHODS FOR FROZEN SECTIONS
1\. Hematoxylin-Eosin method 2. Thionine method 3. Polychrome Methylene Blue method 4. Alcoholic Pinacyanol method
48
New cards
H&E staining of Frozen Sections (Progressive Staining)
Steps:
1\. Harris hematoxylin
2\. Blue in ammonia water
3\. Counterstain with 5% aqueous eosin or 1% alcohol eosin
4\. Dehydrate in increasing concentrations of alcohol
5\. Clear with xylene
● These stains are arranged in Coplin jars so staining for frozen sections will only take about 5-10 minutes
1\. Harris hematoxylin
2\. Blue in ammonia water
3\. Counterstain with 5% aqueous eosin or 1% alcohol eosin
4\. Dehydrate in increasing concentrations of alcohol
5\. Clear with xylene
● These stains are arranged in Coplin jars so staining for frozen sections will only take about 5-10 minutes
49
New cards
Nuclei
blue to blue black
50
New cards
Karyosome
Dark blue
51
New cards
Cytoplasm, proteins in edema fluid
Pale pink
52
New cards
RBCs, eosinophilic granules, keratin
Bright orange-red
53
New cards
Basophil cytoplasm, plasma cells & osteoblasts
Purplish pink
54
New cards
Cartilage
Pink/Light blue to dark blue
55
New cards
Calcium and calcified bone
Purplish blue
56
New cards
Decalcified bone matrix, collagen and osteoid
Pink
57
New cards
Muscle fibers
Deep pink
58
New cards
Mercuric chloride fixed tissue
staining time is increased for hematoxylin while duration of eosin staining should be reduced
59
New cards
chromium and osmium fixed tissues
Staining may be prolonged (H&E staining of Frozen Sections)
60
New cards
Require special stains
Neuroglial fibers, Axon, Nerve endings, Reticulum, Golgi bodies and Mitochondria
61
New cards
COLLODIONIZATION OF SECTIONS
● Ribbons: coating the slide with dilute (thin) celloidin solution
● Recommended for sections that will subjected to strong alkaline or acid solutions that require glycogen demonstration
● This is used for tissues where glycogen should be demonstrated ● Paraffin ribbons containing air bubbles, torn, or inadequately infiltrated sections are likely to float from the side when deparaffinized and stained. The celloidin will be removed in the final dehydration with absolute alcohol before clearing and mounting.
● Recommended for sections that will subjected to strong alkaline or acid solutions that require glycogen demonstration
● This is used for tissues where glycogen should be demonstrated ● Paraffin ribbons containing air bubbles, torn, or inadequately infiltrated sections are likely to float from the side when deparaffinized and stained. The celloidin will be removed in the final dehydration with absolute alcohol before clearing and mounting.
62
New cards
STAINING OF CELLOIDIN SECTIONS
● Uses cellulose nitrate, hence, it is soluble in absolute alcohol ● Sections treated with 95% alcohol are transferred to a mixture of equal parts of chloroform, absolute alcohol and xylene and mounted in Xam
63
New cards
RE-STAINING FADED SLIDES
● Subject to a differentiating agent such as 1-2% alcohol
64
New cards
BROKEN SLIDES
● Employ a mounting media ● Remove the cover slip by soaking in xylene and place in an incubator ● 6 parts Butyl:1 part Durofix
65
New cards
RE-STAINING OF OLD SECTIONS
● Old, Bleached, or Faded sections may be restrained.
1\. Slides are immersed in xylene for 24 hours or gently heated until the mounting medium begins to bubble.
2\. Cover slip may then be removed by lifting it with a dissecting needle.
3\. Section is placed in xylene for 30 minutes to remove the remaining canada balsam and then brought to water.
4\. It is placed in a 0.5% potassium permanganate solution for 5 to 10 minutes, rinse in tap water and subsequently immersed in 5% oxalic acid for 5 minutes or until the section is decolorized.
5\. After washing it again in running tap water for another 5 minutes, the section may then be re-stained with the appropriate staining technique
1\. Slides are immersed in xylene for 24 hours or gently heated until the mounting medium begins to bubble.
2\. Cover slip may then be removed by lifting it with a dissecting needle.
3\. Section is placed in xylene for 30 minutes to remove the remaining canada balsam and then brought to water.
4\. It is placed in a 0.5% potassium permanganate solution for 5 to 10 minutes, rinse in tap water and subsequently immersed in 5% oxalic acid for 5 minutes or until the section is decolorized.
5\. After washing it again in running tap water for another 5 minutes, the section may then be re-stained with the appropriate staining technique
66
New cards
Natural Dyes
● Sources: plants and animals ● Previously utilized for dyeing wool and cotton e.g. cochineal dyes, logwood dyes, and vegetable extracts
NATURAL DYES: 1. Hematoxylin 2. Cochineal dyes 3. Orcein
NATURAL DYES: 1. Hematoxylin 2. Cochineal dyes 3. Orcein
67
New cards
Hematoxylin
● Most valuable for cytologist ● Is NOT a stain itself ● Is natural dye derived by the extraction from the core of the heartwood of a Mexican tree known as “Hematoxylin Campechianum ● It has powerful nuclear and chromatin staining capacity, and its striking polychrome properties which may be produced with proper differentiation. ●It may be used after almost any fixative and is a permanent stain It is frequently used in combination with alum (blue aluminum salt lake), iron (blue black ferric salt lake), chromium and copper salts which act as mordants catalyzing or forming links between hematin stain and the tissue
68
New cards
hematin
active coloring agent, which is formed by the oxidation of hematoxylin, a process known as "ripening."
69
New cards
Natural Ripening
It is usually accomplished by exposing the substance to air and sunlight, thereby oxidizing hematoxylin
* It takes as long as 3-4 months
* It takes as long as 3-4 months
70
New cards
Artificial Ripening
oxidizing agents for ripening converts hematoxylin to hematin almost instantaneously by chemical oxidation so that the staining solution is ready for use immediately after preparation
Oxidizing agents: ● Hydrogen peroxide ● Mercuric oxide ● Potassium permanganate ● Sodium perborate ● Sodium iodate
Oxidizing agents: ● Hydrogen peroxide ● Mercuric oxide ● Potassium permanganate ● Sodium perborate ● Sodium iodate
71
New cards
Cochineal Dyes
● extracted from the female cochineal bug (Coccus Cacti), which is treated with alum to produce the dye, carmine ● is a powerful chromatin and nuclear stain for fresh material and smear preparations
● When combined with:
○ Picric acid (Picroamine): used extensively in neuropathological studies
○ Aluminum chloride (Best’s carmine stain): used for glycogen demonstration
● When combined with:
○ Picric acid (Picroamine): used extensively in neuropathological studies
○ Aluminum chloride (Best’s carmine stain): used for glycogen demonstration
72
New cards
Orcein
● vegetable dye ● derived from certain lichens ● a weak acid, and is soluble in alkali ● normally colorless, but which, when treated with ammonia and exposed to air, produce blue or violet colors
● mainly used for staining elastic fiber
Weak acid; soluble in alkali and is mainly used for staining elastic fibers. Litmus/litmus paper is also obtained from lichens that are treated with lime and soda and exposed to ammonia and air
Not used as a cytological stain because of its poor staining property. It is instead used mainly as an indicator (pH indicator)
● mainly used for staining elastic fiber
Weak acid; soluble in alkali and is mainly used for staining elastic fibers. Litmus/litmus paper is also obtained from lichens that are treated with lime and soda and exposed to ammonia and air
Not used as a cytological stain because of its poor staining property. It is instead used mainly as an indicator (pH indicator)
73
New cards
SYNTHETIC DYES
● known as Coal Tar Dyes
● derived from the hydrocarbon benzene (C6H6) and are collectively known as Aniline dyes
● they were originally manufactured from substances that have been taken from coal tar
● derived from the hydrocarbon benzene (C6H6) and are collectively known as Aniline dyes
● they were originally manufactured from substances that have been taken from coal tar
74
New cards
CHROMOPHORES
● are substances with definite atomic groupings and are capable of producing visible colors ● Simple benzene compounds which contain such substances are known as chromogens ● These are different from the dyes in that any color that they impart to the tissue is not permanent and can, therefore, be easily removed
75
New cards
Auxochrome
● an auxiliary radical or substance which imparts to the compound the property of electrolytic dissociation, thereby altering the shade of the dye, enabling it to form salts with another compound, and ultimately retaining its color
76
New cards
dye
should consist of a chromophore and an auxochrome group attached to a hydrocarbon benzene ring.
77
New cards
Dye modifiers
1\. Ethyl groups 2. Methyl groups 3. Sulfonic groups
78
New cards
ACID DYES
● ACID- active coloring component ● BASE- inactive coloring component ● Example: Picric Acid
● Trichloroacetic acid, picric acid and chromium-fixed tissues usually take in acidic dyes more readily. ● Basic cell structures (collagen, eosinophilic granules of leukocytes, etc.) have an affinity for the acid dye ions and are regarded as acidophilic.
● Trichloroacetic acid, picric acid and chromium-fixed tissues usually take in acidic dyes more readily. ● Basic cell structures (collagen, eosinophilic granules of leukocytes, etc.) have an affinity for the acid dye ions and are regarded as acidophilic.
79
New cards
Picric Acid
○ Fixes, differentiate, and stain tissues ○ For Van Gieson’s stain for connective tissue ○ For microscopic study of fungi
80
New cards
acidophilic
Basic cell structures (collagen, eosinophilic granules of leukocytes, etc.) have an affinity for the acid dye ions
81
New cards
BASIC DYES
● Base- active component
● where the active coloring substance is found in a basic component that combines with the acid radical (usually taken from sulfuric, acetic or hydrochloric acid)
● Examples:
○ Methylene blue- used as an indicator and a dye; basic nuclear stain
○ Mercuric chloride and formaldehyde fixed tissues- favor staining with basic dyes
● Acidic cell structures (chromatin, mucus, cartilage matrix etc.) have an affinity for basic dye ions and are therefore regarded as basophilic
● where the active coloring substance is found in a basic component that combines with the acid radical (usually taken from sulfuric, acetic or hydrochloric acid)
● Examples:
○ Methylene blue- used as an indicator and a dye; basic nuclear stain
○ Mercuric chloride and formaldehyde fixed tissues- favor staining with basic dyes
● Acidic cell structures (chromatin, mucus, cartilage matrix etc.) have an affinity for basic dye ions and are therefore regarded as basophilic
82
New cards
basophilic
Acidic cell structures (chromatin, mucus, cartilage matrix etc.) have an affinity for basic dye ions
83
New cards
NEUTRAL DYES
● combination of aqueous solution of acid and basic dyes ● stains cytoplasm and nucleus simultaneously and differentially ● insoluble/barely soluble in water ● soluble in alcohol
● Examples: Romanowsky dye, Giemsa stain, Irishman’s stain
● Examples: Romanowsky dye, Giemsa stain, Irishman’s stain
84
New cards
COMMON STAINING SOLUTIONS USED
1\. Hematoxylin
2\. Eosin
3\. Acid Fuchsin-Picric Acid
4\. Acridine Orange
5\. Acridine Red 3B
6\. Alcian Blue
7\. Aniline Blue
8\. Basic Fuchsin
9\. Benzidine 1
10\. Bismarck Brown
2\. Eosin
3\. Acid Fuchsin-Picric Acid
4\. Acridine Orange
5\. Acridine Red 3B
6\. Alcian Blue
7\. Aniline Blue
8\. Basic Fuchsin
9\. Benzidine 1
10\. Bismarck Brown
85
New cards
ALUMINUM HEMATOXYLIN SOLUTIONS
● Recommended for progressive staining ● gives a “blue-lake”, increase the selectivity for nuclei
● Ripening agents:
○ Ehrlich’s: Sodium iodate ○ Harris’s: Mercuric chloride
● During staining, these are passed on to an alkaline solution to neutralize the acid and free OH group, to form an insoluble blue aluminum hematin-tissue lake
● Acid solutions- red ● Alkaline solutions- 1% hydroxide
● Ripening agents:
○ Ehrlich’s: Sodium iodate ○ Harris’s: Mercuric chloride
● During staining, these are passed on to an alkaline solution to neutralize the acid and free OH group, to form an insoluble blue aluminum hematin-tissue lake
● Acid solutions- red ● Alkaline solutions- 1% hydroxide
86
New cards
ALUMINUM HEMATOXYLIN SOLUTIONS
Ehrlich’s Hematoxylin
Harris Hematoxylin
Cole’s Hematoxylin
Mayer’s Hematoxylin
Harris Hematoxylin
Cole’s Hematoxylin
Mayer’s Hematoxylin
87
New cards
BLUEING
● The process where alum hematoxylin stained sections are passed on to an alkaline solution in order to neutralise the acid and free the OH group, to form an insoluble blue aluminum hematin-tissue-lake
● For alum-hematoxylin-stained sections: warm (40° to 50°C) tap water is commonly used, since it is generally sufficiently alkaline (5 to 15 minutes)
● When tap water is not sufficiently alkaline, or is even acid, and is unsatisfactory for blueing hematoxylin, use:
○ Lithium carbonate (30 seconds) - form crystalline deposit
○ Bicarbonate
○ Potassium or sodium acetate
○ Scott’s Tap Water Substitute (60 seconds)
○ Ammonia water (15 seconds)
■ hard on delicate tissues ■ causes tissue to fall off the slide
● The use of very cold water slows down the process while warming accelerates it. In fact, the use of very cold water (below 10°C) for blueing sections may even produce pink artifact discolorations on the tissue
● For alum-hematoxylin-stained sections: warm (40° to 50°C) tap water is commonly used, since it is generally sufficiently alkaline (5 to 15 minutes)
● When tap water is not sufficiently alkaline, or is even acid, and is unsatisfactory for blueing hematoxylin, use:
○ Lithium carbonate (30 seconds) - form crystalline deposit
○ Bicarbonate
○ Potassium or sodium acetate
○ Scott’s Tap Water Substitute (60 seconds)
○ Ammonia water (15 seconds)
■ hard on delicate tissues ■ causes tissue to fall off the slide
● The use of very cold water slows down the process while warming accelerates it. In fact, the use of very cold water (below 10°C) for blueing sections may even produce pink artifact discolorations on the tissue
88
New cards
Ehrlich’s Hematoxylin
● naturally ripened ● Blue: cartilage and cement lines of bones
● Glycerin
○ Stabilizer
○ retards evaporation of the solution
○ slows down ripening
● recommended for tissues that have become acidic
● mainly used for regressive staining and not ideal for frozen sections
● This naturally ripening alum hematoxylin takes about 2 months to ripen, but its staining property will last for months or years
● Glycerin
○ Stabilizer
○ retards evaporation of the solution
○ slows down ripening
● recommended for tissues that have become acidic
● mainly used for regressive staining and not ideal for frozen sections
● This naturally ripening alum hematoxylin takes about 2 months to ripen, but its staining property will last for months or years
89
New cards
Harris Hematoxylin
● is widely used for routine nuclear staining, exfoliative cytology, staining sex chromosome ● should assume a dark purple color when ripened with mercuric oxide
● 4% glacial acetic acid: give more precise nuclear staining
● 10mL of ethanol: prevents mold growth
● A good regressive stain ● Best results are obtained when the solution is made every 2 or 3 months
● 4% glacial acetic acid: give more precise nuclear staining
● 10mL of ethanol: prevents mold growth
● A good regressive stain ● Best results are obtained when the solution is made every 2 or 3 months
90
New cards
Cole’s Hematoxylin
● recommended for routine purposes ● used in sequence with Celestine blue ● artificially ripened with an alcoholic iodine solution ● ready for immediate use ● Staining time: 10 minutes
91
New cards
Mayer’s Hematoxylin
● chemically ripened with sodium iodate ● is a nuclear counterstain to demonstrate cytoplasmic glycogen ● used in acid-alcohol differentiation ● used in Celestine Blue hemalum method ● both a regressive and progressive stain ● Chloral hydrate is added to the final solution as preservative ● Disadvantage: can be stored only for 3-6 months at the most
92
New cards
IRON HEMATOXYLIN SOLUTIONS
● Used only for differential or regressive staining ● Using acid-alcohol as a differentiating agent
1\. Weighert’s Hematoxylin Solution
2\. Heidenhain’s Hematoxylin Solution
1\. Weighert’s Hematoxylin Solution
2\. Heidenhain’s Hematoxylin Solution
93
New cards
Weigert’s Hematoxylin Solution
● Mordant/oxidant: ferric ammonium chloride ● Color: ○ deep purple blue-black-violet, through violet, purple, ○ brown and yellowish brown within 2 to 3 weeks ● used for demonstrating muscle fibers and connective tissues ● standard iron hematoxylin stain used ● A solution that has turned brown should be discarded
94
New cards
Heidenhain’s Hematoxylin Solution
● Mordant/oxidant: ferric ammonium sulfate ● is a cytological stain recommended for regressive staining of thin sections ● color: black or dark grey ● used to demonstrate: chromatin, chromosomes, nucleoli, centrosome, and mitochondria. ● Voluntary muscle striations and myelins are also well stained
95
New cards
Phosphotungstic Acid Hematoxylin (PTAH)
● mordant: 1% aqueous phosphotungstic acid ● immediate ripening: adding 50mL of 0.25% K permanganate
● color:
○ reddish brown to purple
○ **blue**: nuclei, fibrin, muscle striations, myofibrils and fibroglia
○ **orange-red/brownish red to brick red stain**: collagen, bone and cartilage
● demonstrates in paraffin, celloidin and frozen sections
● peak staining activity is not reached until after 7 days
● a progressive stain ● staining time: 12-24 hours
● color:
○ reddish brown to purple
○ **blue**: nuclei, fibrin, muscle striations, myofibrils and fibroglia
○ **orange-red/brownish red to brick red stain**: collagen, bone and cartilage
● demonstrates in paraffin, celloidin and frozen sections
● peak staining activity is not reached until after 7 days
● a progressive stain ● staining time: 12-24 hours
96
New cards
EOSIN
● used for staining connective tissues and cytoplasm ● used as a counterstain after hematoxylin and before methylene blue ● background stain: pleasing and colorful contrast
97
New cards
1\. Yellowish (Eosin Y)
a. Most commonly used
b. Gives green yellow fluorescence in alcoholic medium
c. Readily soluble in water and less in alcohol
b. Gives green yellow fluorescence in alcoholic medium
c. Readily soluble in water and less in alcohol
98
New cards
Bluish- deeper red color
(Eosin B, Erythrosin B)
99
New cards
Ethyl Eosin- Eosin S,
eosin alcohol-soluble
100
New cards
Acid Fuchsin-Picric Acid (Van Gieson’s stain
○ demonstration of connective tissues