[CHEM] Quantum Numbers & Electron Configuration
1/17
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
quantum numbers
n (principal quantum number), l (angular momentum quantum number), ml (magnetic quantum number), ms (spin quantum number)
n
principal quantum number; represents the energy level of an electron in an atom in positive integers
l
angular momentum quantum number/azimuthal quantum number; determines the shape of the orbital; numbers anywhere from 0 → (n-1)
ml
magnetic quantum number; describes the orientation of the orbital’s shape; numbers anywhere from -l … l
ms
spin quantum number; describes the spin orientation of the electron; values -1/2 or 1/2
l = 0
s orbital
l = 1
p orbital
l = 2
d orbital
l = 3
f orbital
electron configuration
notation that describes the distribution of electrons among the orbitals of an atom
s subshell orbital count
one orbital which holds two electrons
p subshell orbital count
three orbitals which holds six electrons (two each)
d subshell orbital count
five orbitals which holds ten electrons (two each)
f subshell orbital count
seven orbitals which holds fourteen electrons (two each)
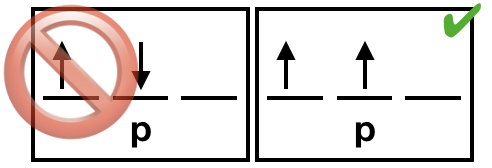
Pauli exclusion principle
no two electrons in the same atom have the same four quantum numbers (i.e., a. no more than two electrons in the same orbital and b. electrons in the same orbital have opposite spin values).
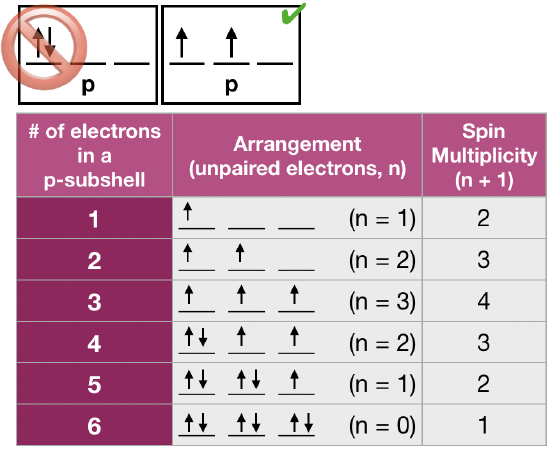
Hund’s rule
the lowest energy arrangement is the one with the greatest number of unpaired electrons; every orbital in a sublevel is occupied by one electron before electrons double up in an orbital
Aufbau principle
electrons fill orbitals from lowest-energy to highest-energy
exchange interaction
ground state electron configuration will have all unpaired electrons having the same spin (written typically in “spin up”)