H 1.3 Redox
1/28
Earn XP
Description and Tags
Flashcards covering key vocabulary from the 'Unit 1: Chemical Changes and Structure, 1.3 Redox' lecture, including definitions of oxidation, reduction, agents, electrochemical series, and titration concepts.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|

29 Terms
Reduction
A gain of electrons by a reactant in any reaction.
Oxidation
A loss of electrons by a reactant in any reaction.
Redox Reaction
A chemical reaction in which reduction and oxidation take place at the same time.
Oxidising Agent
A substance that accepts electrons, causing another substance to be oxidised, and is itself reduced. (strongest at the bottom left side of the electrochemical series, the strength decreases as you go up the series, strongest in group 7 elements, high electronegativities, non-metals)
Reducing Agent
A substance that donates electrons, causing another substance to be reduced, and is itself oxidised. (strongest at the top right side of the electrochemical series, the strength decreases as you go down the series, strongest in group 1 elements, low electronegativities, metals)
Common reducing agent
Carbon monoxide (CO). A gas that is widely used in blast furnaces to reduce iron from iron ore.
Common Oxidising Agents and their uses
Hydrogen peroxide (H2O2,), permanganate ion (MnO4-) and dichromate ion (Cr2O72-). Uses: Killing fungi and bacteria, inactivating viruses, and breaking down coloured compounds (bleaches).
Volumetric Analysis
A method using a solution of accurately known concentration (standard solution) in a quantitative reaction to determine the concentration of another substance.
Indicator
A substance, usually changing colour, used to show when the end-point of a titration is reached.
Concordant Titre Volumes
Titre volumes that are within 0.2 cm3 of each other, used for calculating the average volume.
Standard Solutions
Solutions of accurately known concentration.
Self-Indicating Reaction
A reaction where a reactant changes colour at the end-point, making an external indicator unnecessary, e.g., acidified permanganate turning colourless.
Transfer Quantitatively
The process of ensuring all of a solution, along with its rinsings, is moved from one container to another, typically to a volumetric flask.
Steps of balancing redox equations
Balance elements other than O and H
Balance O by adding water molecules on the other site of the equation
Balance H
Balance chargers (must be the same of both sides of the equation)
Half-equation
Reduction or oxidation reaction showing the electrons.
How to find the oxidation (or reduction) reaction (half equation) from the balanced redox reaction?
Identify reduction and oxidation (usually one can be easily identified). Extract the half equation and add electrons to show the oxidation (or reduction).
Identify the oxidising agent:
Zn(s) + Cu2+(aq) ⟶ Zn2+(aq) + Cu(s)
Cu2+ (accepts electrons from Zn)
note the decrease in charge due to electrons gained
Identify the reducing agent:
Zn(s) + Cu2+(aq) ⟶ Zn2+(aq) + Cu(s)
Zn (donates electrons to Cu2+)
note the increase in charge due to electrons lost
When copying equations from the ECS, which one gets reversed?
Higher one gets reversed (this is your oxidation reaction)
What’s wrong with this redox equation?
Ni(s) + SO42−(aq) + 2H+(aq) + H2O(l) ⟶ Ni2+(aq) + SO32−(aq) + H2O(l) + e–
Any of:
Still has electrons/charges not balanced
Water present on both sides
What must be done before these equations can be added up?
Na(s) ⟶ Na+(aq) + e−
Cl2(g) + 2e− ⟶ 2Cl−(aq)
Electrons must be balanced (top equation x2)
(This is done to balance the charges in the redox equation.)
How do you finish writing this type of equation?
Sn3+ ⟶ Sn2+
Add required no. of electrons to the more positive side.
Sn3+ + e− ⟶ Sn2+
Dichromate and permanganate ions are strong oxidising agents in ________ solutions.
Acidic
Why the solution must be acidified for the reaction with permanganate ion?
to provide H+ ions.
Steps of preparing standard solution
* dissolve solid in small volume of water
* add to volumetric flask with rinsings
* fill up to mark with water
* stopper and invert.
If permanganate is used as a titrant, what is the colour change seen during the titration (as the endpoint is reached)?
Colourless to purple
(permanaganate ions are purple, but before the endpoint they will keep getting converted into colourless Mn2+ ions)
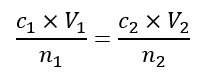
Where do n1 and n2 come from?
Balanced equation - NOT calculated using n=cV!
Suggest what you would use to accurately measure 10 cm3 of solution
A pipette
What indicator should be used in a titration involving iodine?
Starch