L3: Layer-specific Targeting and Topographic Maps
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
65 Terms
Why are topographic maps needed
so the brain can integrate a multitude of stimuli from the environment
formed in the sensory and motor areas
Discovery example of somatotopy 1
John Langley: Reflex-mediating neurons of the superior Cervical ganglion Topographically ordered:
If activate neurons with axons projecting ot of the top thoracic nerve root→ pupil reflexb
If activate neurons with axons exiting the fourth thoracic nerve root→ Dilation and constriction of the blood vessels of the ear
THEREFORE: an order→ topogrphical map
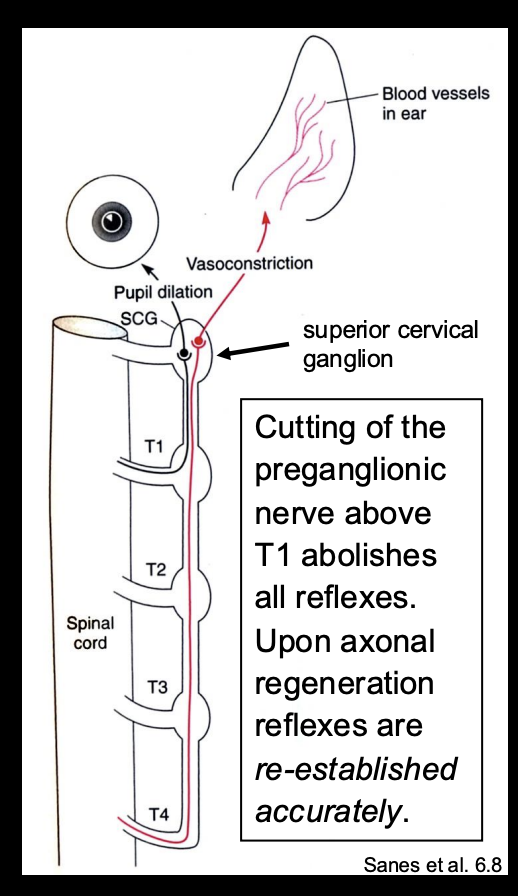
What did Langley also find
Cutting of the preganglionic nerve above T1→ abolishes all reflexes
Upon axonal regneration→ reflexes are re-established eccuratley
What this suggests→ the region itself has some kind of order→ somehow marked and find eachother again to be regenerated
prior order is restablished
Discovery example of topography 1
Wilder PEnfield
Used electrodes to systematically probe different areas of the cortex of patients who were consicous during the operation
result:
Found motor and sensory cortical areas
BOTH organised to for somatotopic maps
Neural maps what are they
internal representations of the body and/or the outside world
Maps can be discrete or continuous
Neural maps where can they be found
found throughout the nervous system
for sensory→ auditory, visual, olfactor maps etc
for motor systems
Why are they useful
constitute a fundamental organising principle
strategy for organising and presenting synaptic information
facilitate complex neuronal wiring of populations of neurons by providing:
order to the spatial relationships
and/or
qualitative relationships
Types of maps
Spatial
preservation of the nearest neighbour
anatomical representation within the brain
e.g Homunculus→ nearest relastionship of the body represented
Quality map
e.g types of smell or taste
widley distributed over the receptors (epithelium)
but the terminals cluster together in a functional sense→ depending on quality
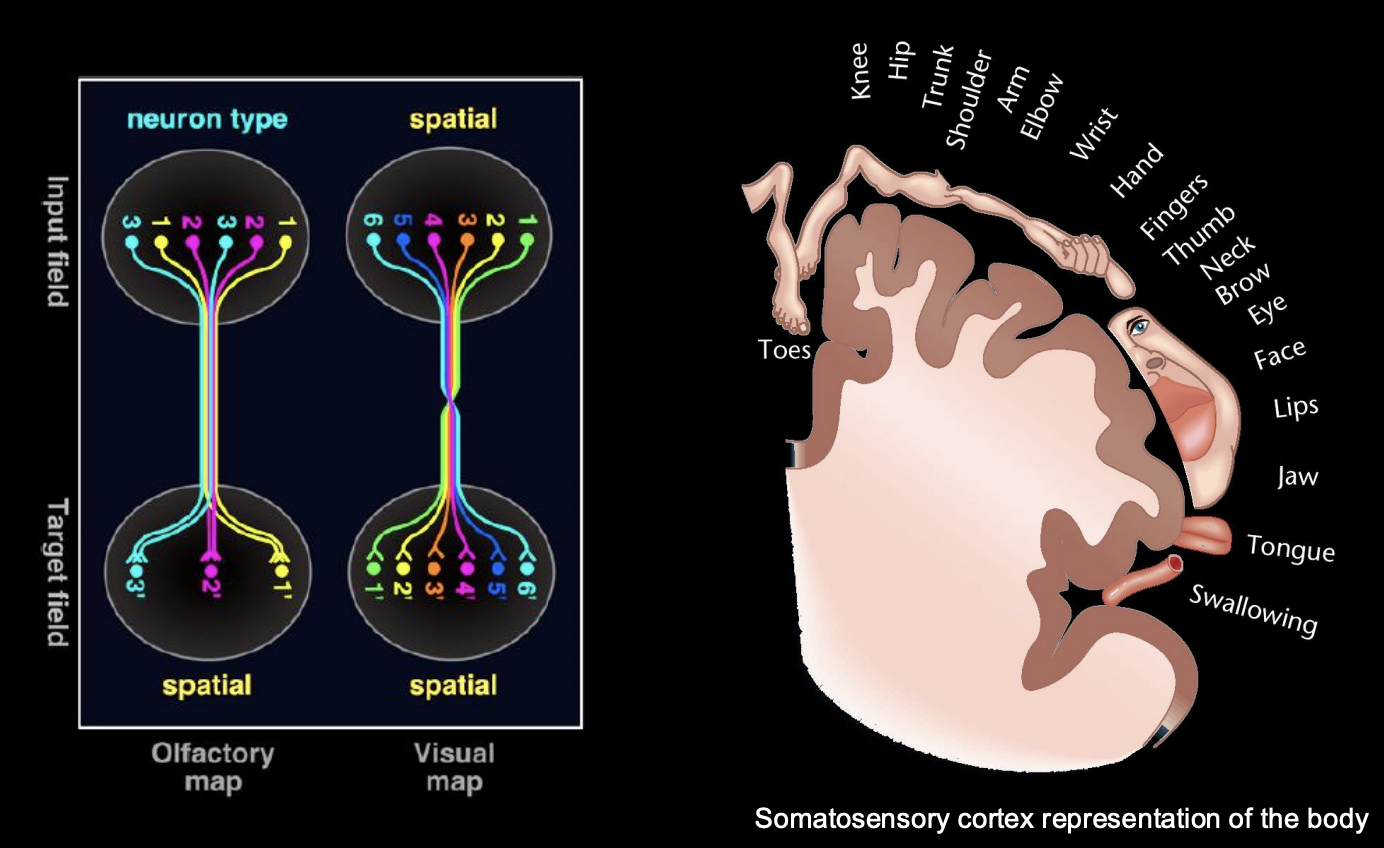
Example of targeting axons to discrete regions→ example
The Motor system
The vertebrate neuromuscular system→ overview of how cell types specify
axon pathfinding seen as a sequence of simple choices
progressively define axon tragetories and target areas
restrict the developmental potential each time and become specified
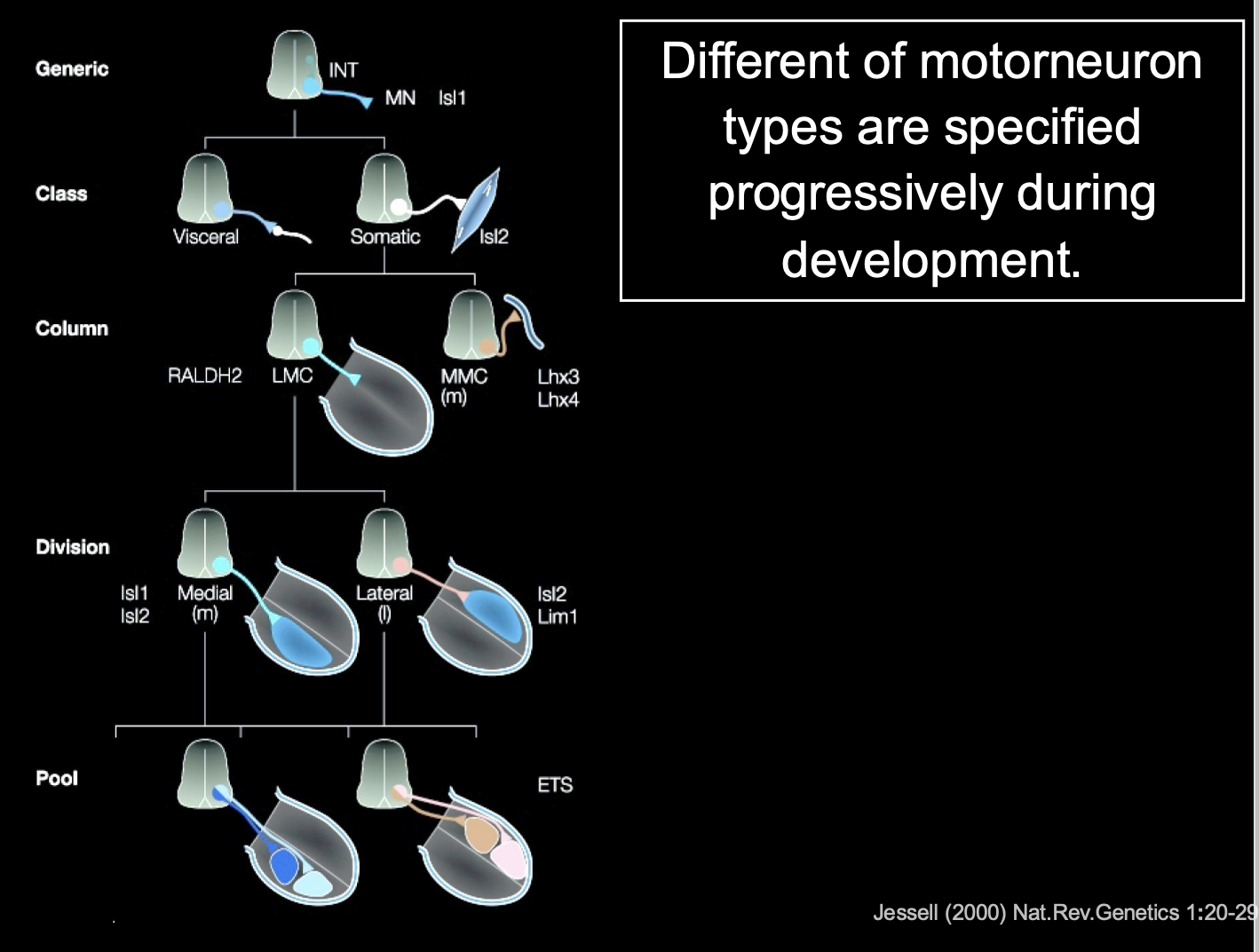
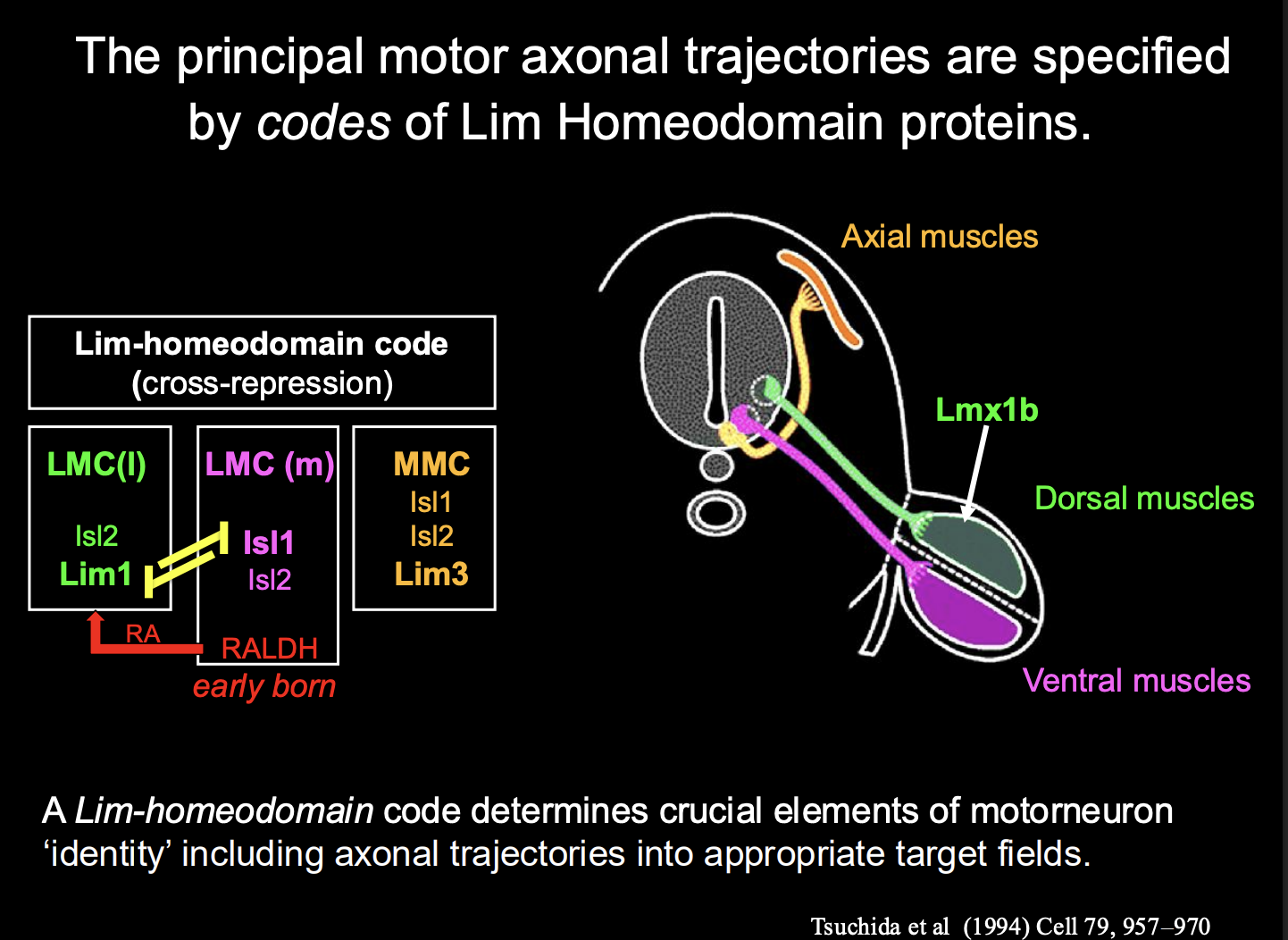
What is this decision process in vertebrate motorneurons
Each dscision is binary
Start off generic
choose visceral or somatic
somatic→ LMC (limb) or MMC
LMC→ medial or lateral
contiues
How is the decision between motoneurons that innervate dorsal limb and those for ventral limb made
Dorsal limb musculature→ located in the lateral part of the LMC)
ventral limbs muscles→ located in the medial part of the LMC
Distinction between these is generated by birth order:
mLCM are born first→ Express the
Lim-Homeodomain (contains both LIM and Homeobox motifs) Transcription factor Islet1
and synthetic enzyme Retinaldehyde Dehydrogenase-2 (RALDH2)
mLCM make RA (retinoic acid)
so the later born lLMC neurons are exposed to RA
causes lLMC to express different transciption factor→ Lim1
causes different surface proteins
So cluster together in different lumps
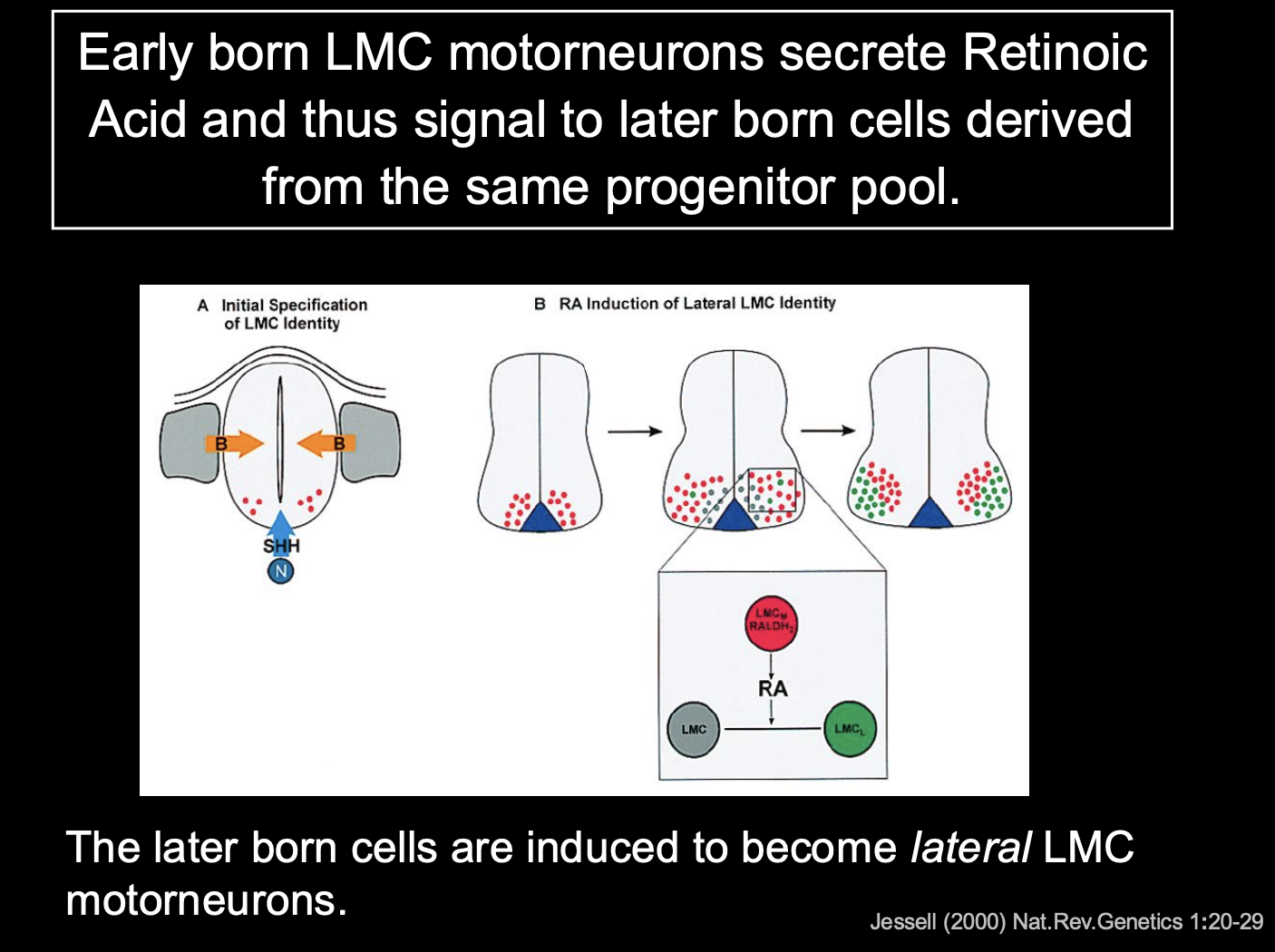
What maintains the identity of these two seprate sets of cells
→ Cross-repressive interactions between islet-1 and Lim-1 maintain a stable distinction between these two sets of cells
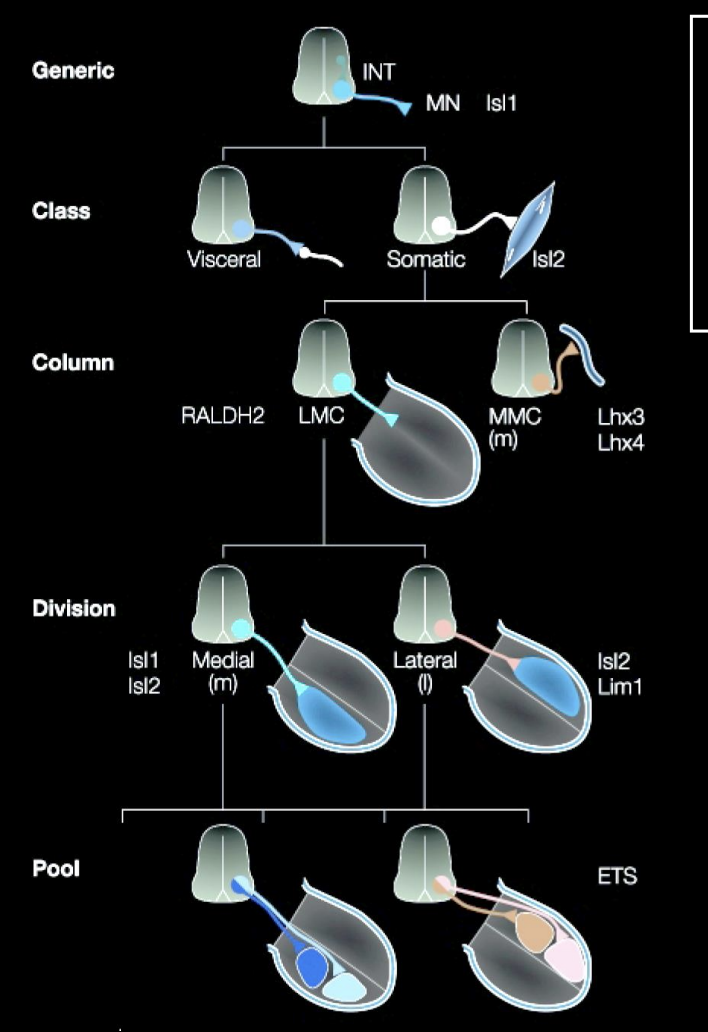
To make Motoneurons innervating axial muscles (MMC in stead of the LMC)
Different Lim-Homeobox transciption factor→ Lim-3 (aka Lhx-3)
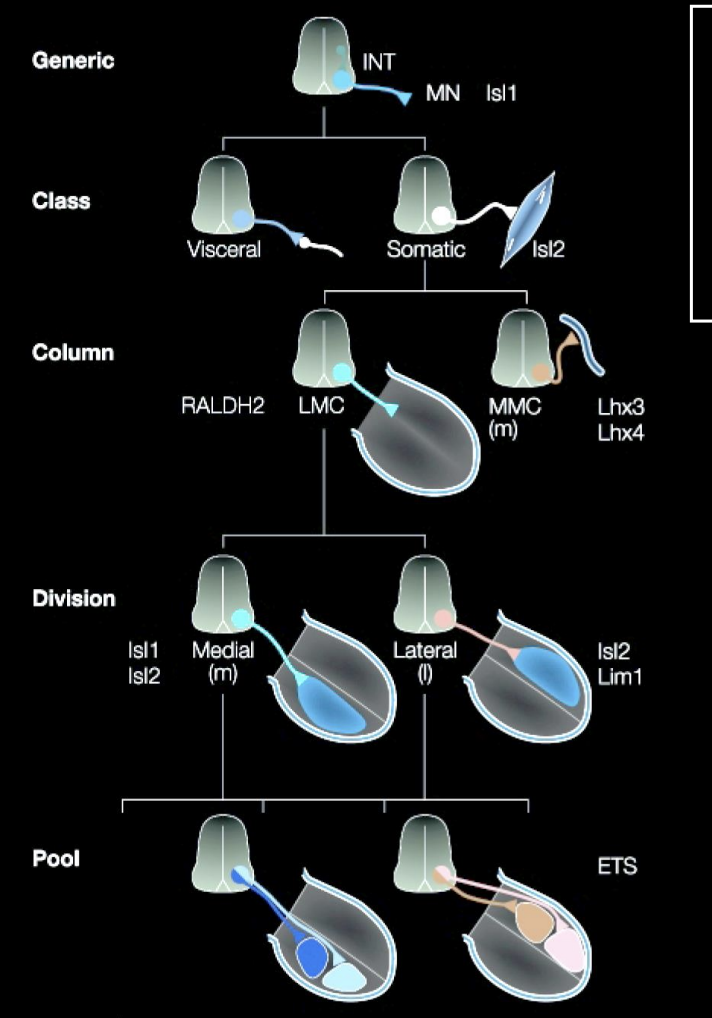
Overall what does this image show how cell identity is made
→ Expression of different combinations (codes) of lim-homeodomain transcription factors
therefore: it is not a single TF that is involved→ kind of a combination
this is useful because it allows great diversity to be made with a small molecular code
(can see in the image that there is repeated use for the same TFs→ but just different codes)
Rather than having a TF expressed for every single different neuron
These codes are made due to birth order
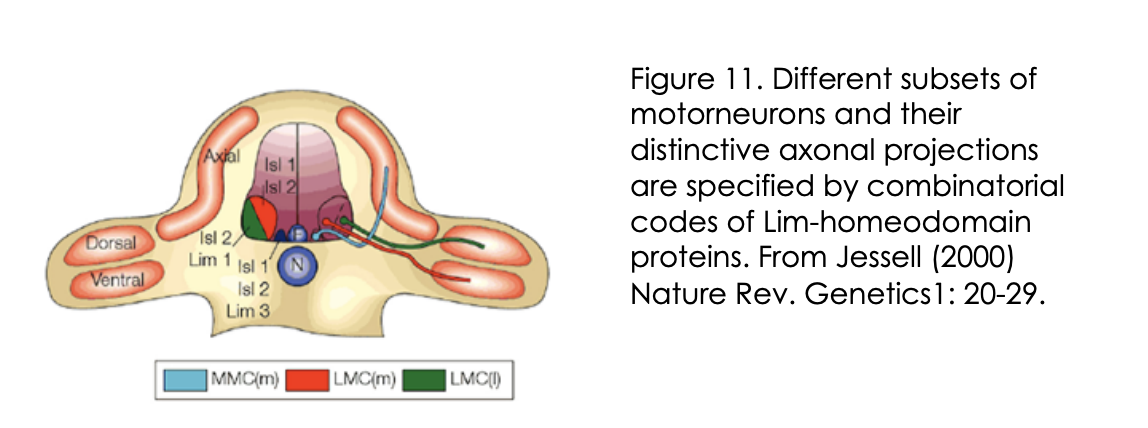
What do these Lim-homeobox TFs also specify for on top of motor neuron identity and how found
Specify distinct axonal trajectors
into distinct target regions in the periphery
How found
Knockout/knockdown and mis-expression of these the lim-homeobox TFs in mice and chick
This axonal specification may be conserved→ evidence
Compared to the vertebrates→ Drosophila embryo combinatorial code
Also for Lim-Homeobox transciption factors:
Islet and Lim3
Motor axon trjectories are also specified
by POU domain factor→ Drifter
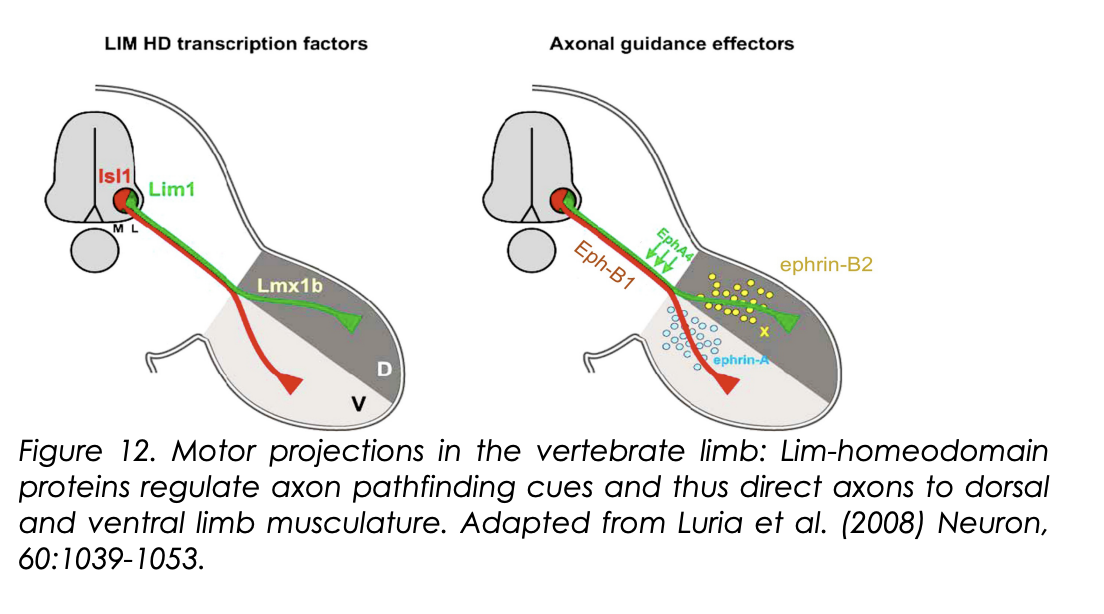
How do these Lim-Homeodomain codes actually cause their effect on identity?
Lim-Homeodomain codes regulate Eph-receptor expression
Islet-1→Eph-B1
Lim-1→Eph-A4
What do these receptors do
Regulate the simple binary choice of whether axons innervate the dorsal or ventral limb
How→ be responding to different gradients of guidance cues found in the limb? (ephrin-A and ephrin B2)
mLMC→Islet-1→Eph-B1→ ephrin-B2 ATTRACTION→ ventral limb
lLMC→Lim-1→Eph-A4→ EphrinA INTERACTIONS→ dorsal limb
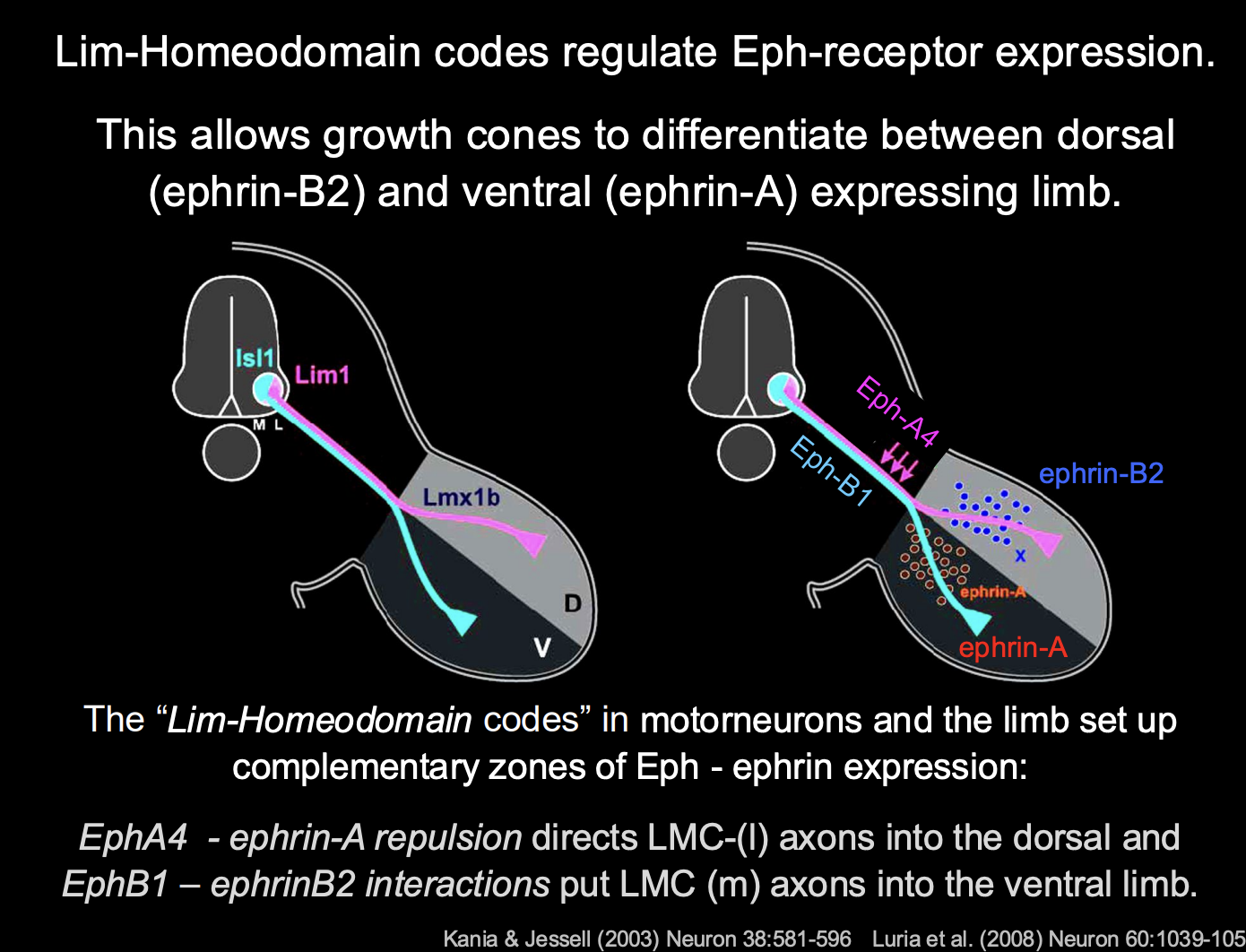
How do these guidance cues work for vertebrate limb
Guidance cues are in complementary zones in the limb:
Ephrin A→ in the ventral
Ephrin B2→ Dorsal
lLMC→ Eph-A4→ repelled by Ephrin A→ goes to the dorsal
mLMC→ Eph-B1 receptor→ interactis with ephrin B2→ goes to ventral
Complemntary zones of Eph-ephrin expression
How is the high fidelity of the neuromuscular connectivity achieved?
employing mutliple of these targeting mechanisms simultaneously
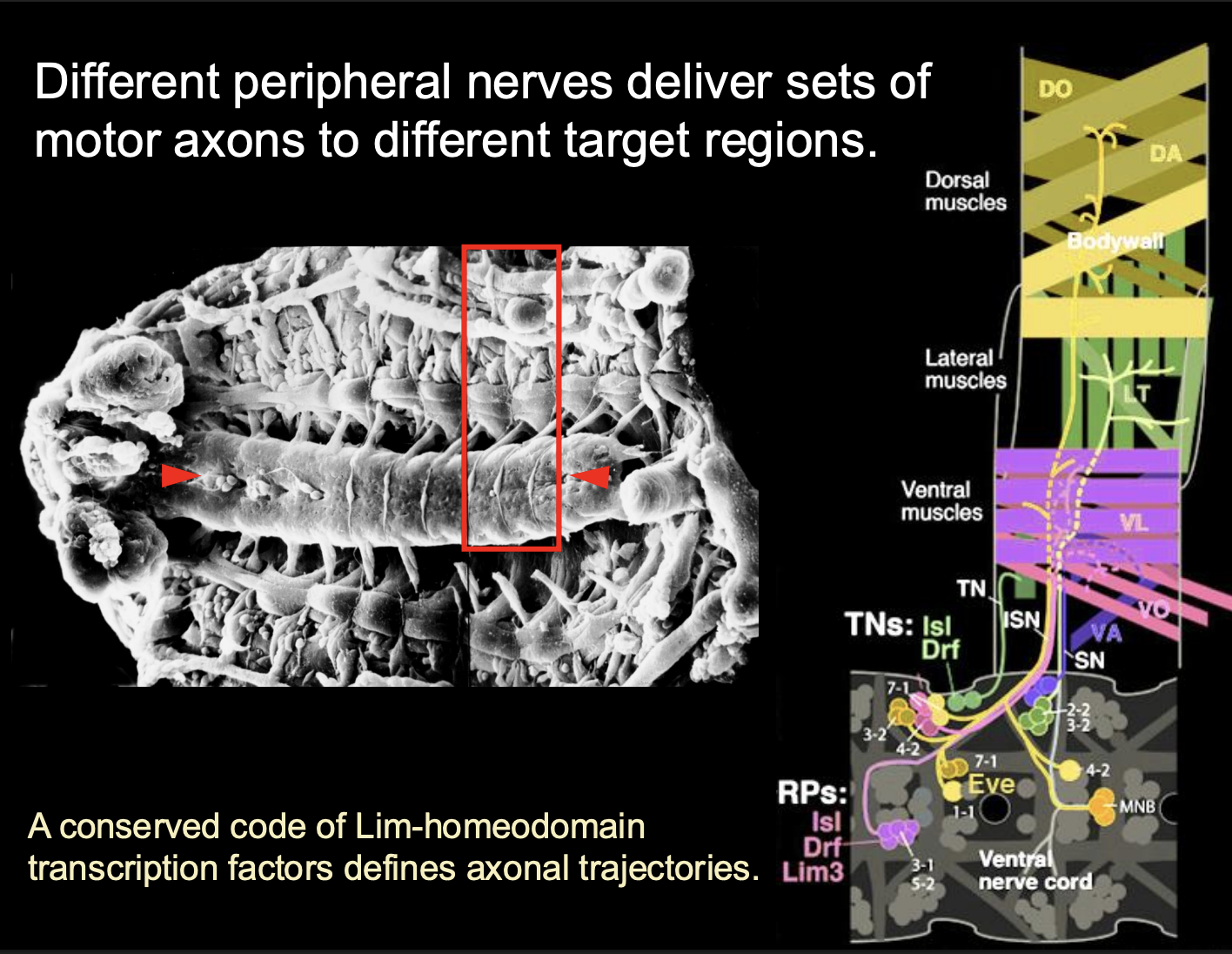
Invertebrate Neuromuscular system→ Drosophila structure overview
30 motor neurons to 30 muscules
1:1 innervation
different peripheral nerves deliver sets of motor axons to different target regions
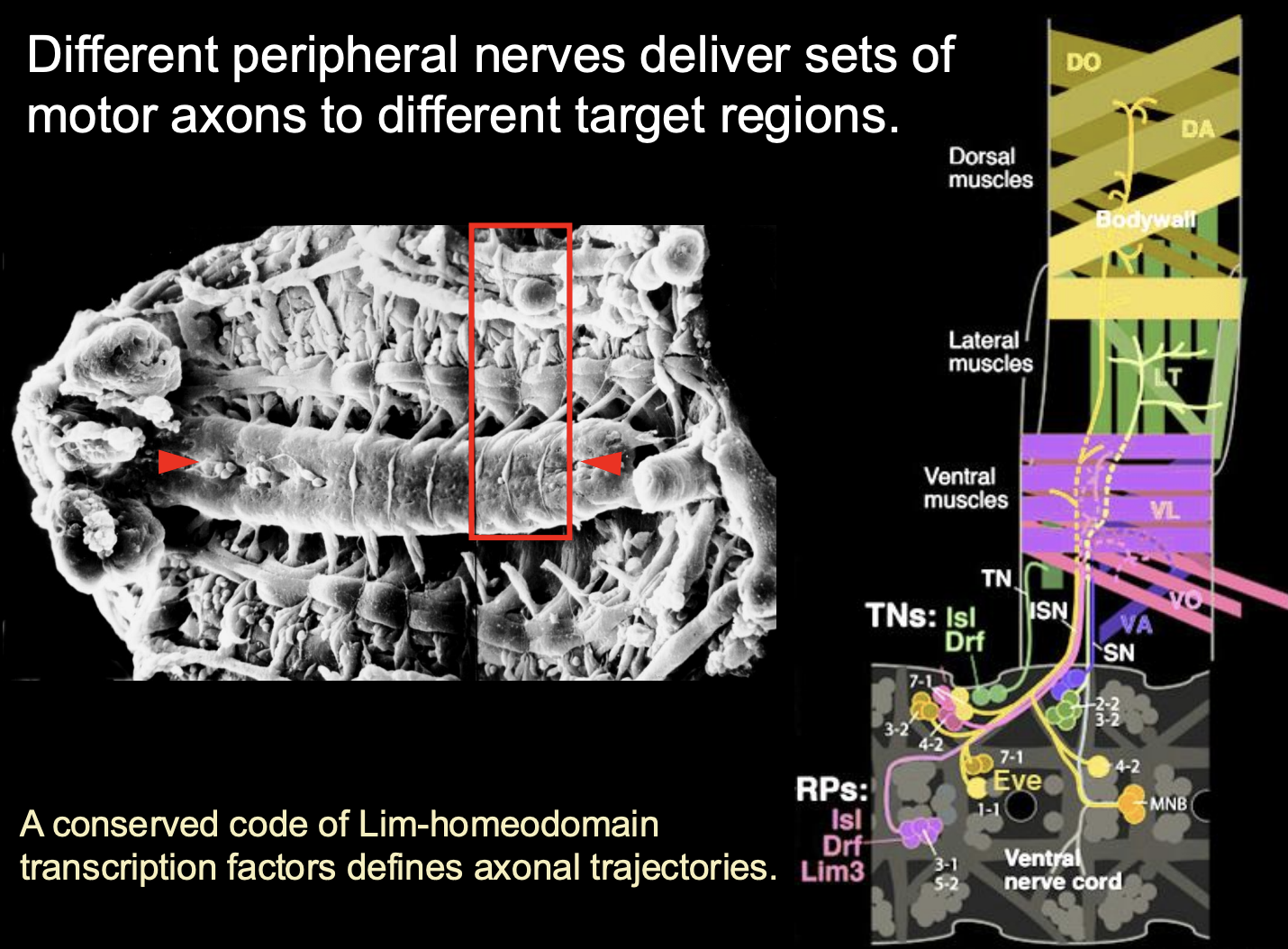
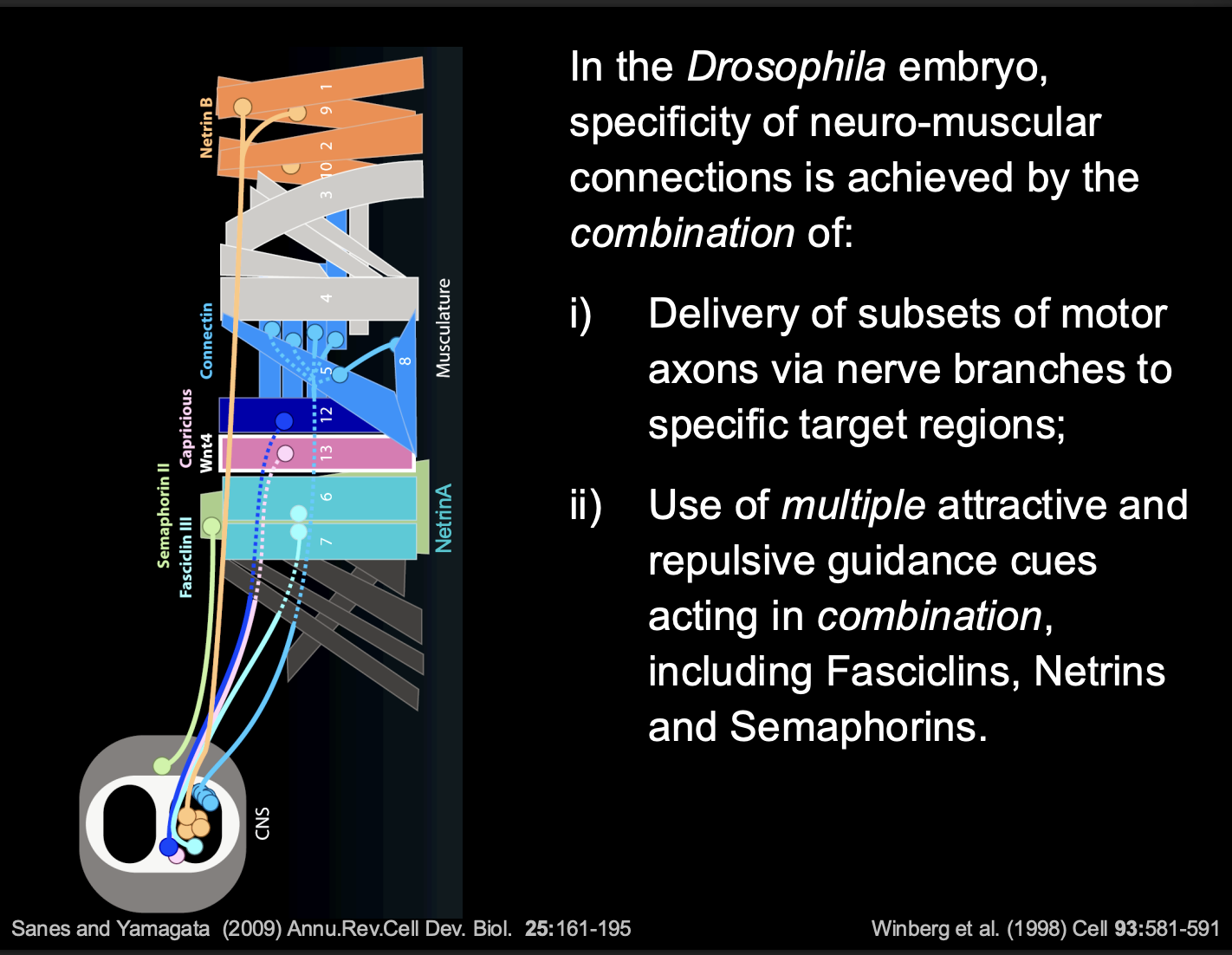
How is this mapping set up?
sets of muscles and their innervating motor axons express matching homophilic Cell Adhesion Molecules (CAMs)
e.g Fascicilin-3 and Connectin
Also, some muscles also express secreted guidance cues
e.g netrins or semaporins
What the combination of these things do
guidance cues modulate the CAM-mediated attraction
And thus→modulate overall balance of attractive and Repulsive forces
growth cone picks up on a there differences and makes a decision
overall: determines the choice of Target muscle
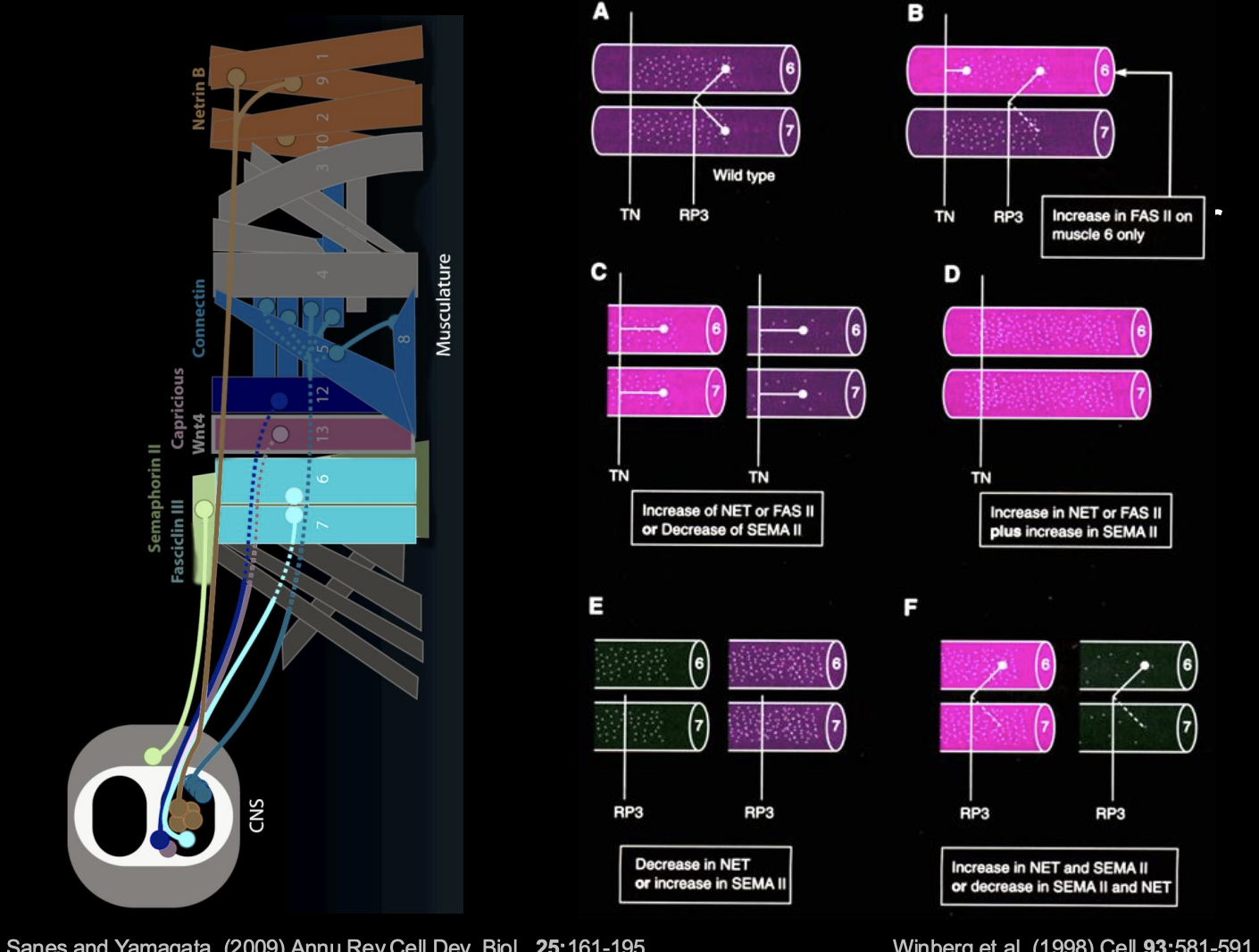
Investigating how these choices are made
Altering the combinatorial code
Procedure:
Change the concentrations of NET and SEMA
see where the neurons project to
can see how the balance of forces due to NET and SEMA change the course of the pathway
Overall summary of the organisation of the neuromuscular system
growth cone guidance of motorneurons mirror the process of cell fate decisions→ hierachical sequence of binary decisions → specificity
Different TPs→ different guidance cue receptors→ different response to existing guidance cues
Integrate musliple guidance cues (secreted and membrane bound)
balance of repulsive and attractive
→ overall confer preferences and specificity to the type and area of the motor neuron

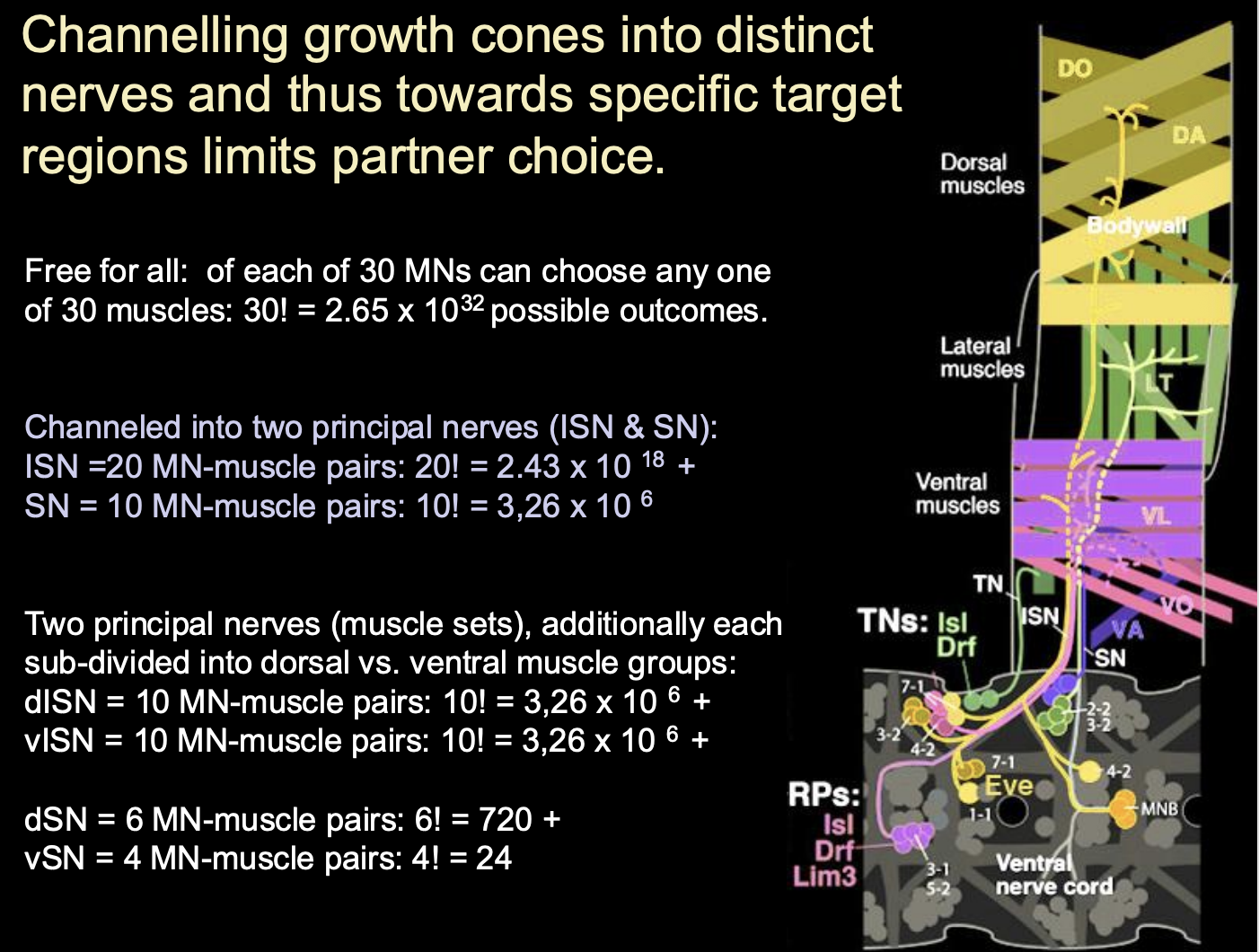
Note: Channelling growth cones into distinct nerves and thus towards specific target regions limits what
→ Partner choice
If you channel a different number of motorneurons- >you will get different choices and outsomes
chanelling is about restriction of choices
Therefore just remember that: although the TFs, receptors and their responses to guidance cues has an impact on the identity, you also have the factor that with every choice made, another neuron’s choice will also be effected. The number of neurons available will mean that the combinatorial codes will become different outcomes?
i.e all affect eachother?
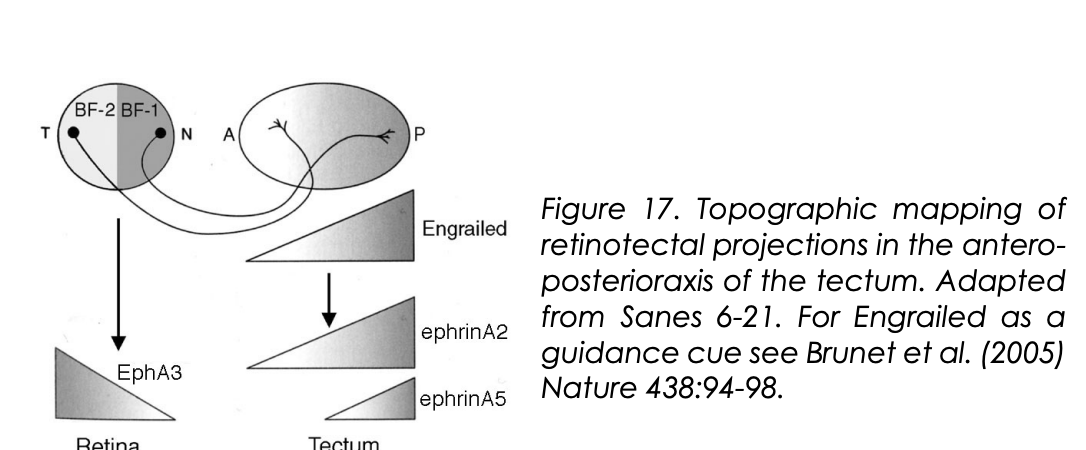
Example of continuous maps
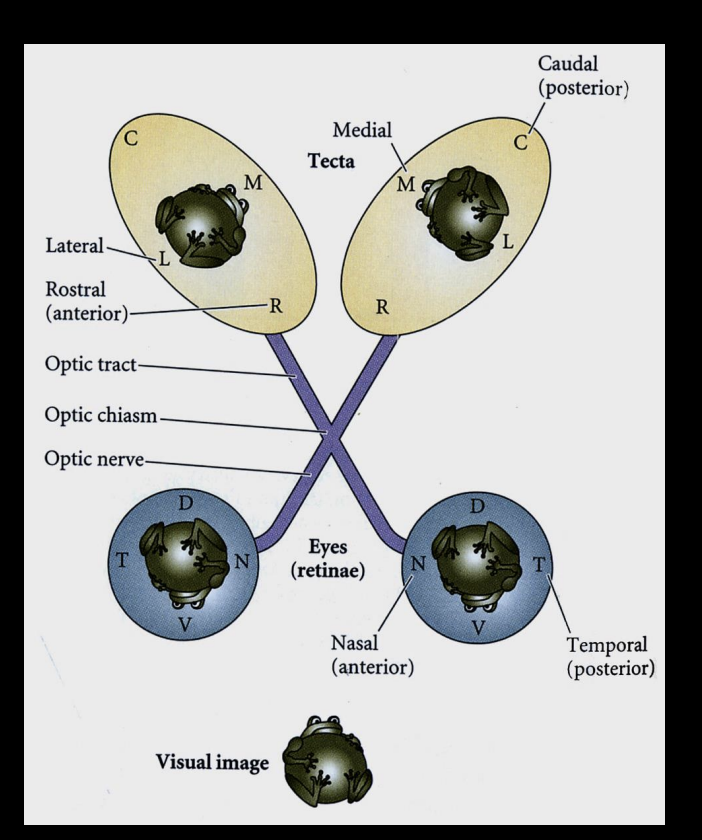
retinotopic projections
unlike motor system→ not 1:1 mapping
How are continuous neural maps formed?→ e.g the Retinotopic map
retinal axons→ optical tectum (project through retinal ganglion cells)
relative position on retina is conserved on the tectum:
Temporal retina→ Anterior tectum
Nasal Retina→ Posterior tectum
: retinal ganglion cells project ro specific regions of the tectum so that neighbouring cells in the retina will form connection next to each other in the tectum, with neighbouring tectal partner neurons
.I.e map of visual space is preserved in the brain
Before the mid 1900, what was thought as to how thse connections were set up?
outcome of trial and error
with functional validation
sorted neural connections according to function
Roger Sperry experiments to challenge this view
Procedure 1:
rotated eyes of a frog by 180 degrees (took eye out and back in)
left optic nerve intact
test behaviour of animal
Result:
frog saw world upside down
Procedure 2: (done to test the re-generative effect)
rotated frog eye 180 degrees
cut optic nerve
Result:
saw world upside down
irreversible
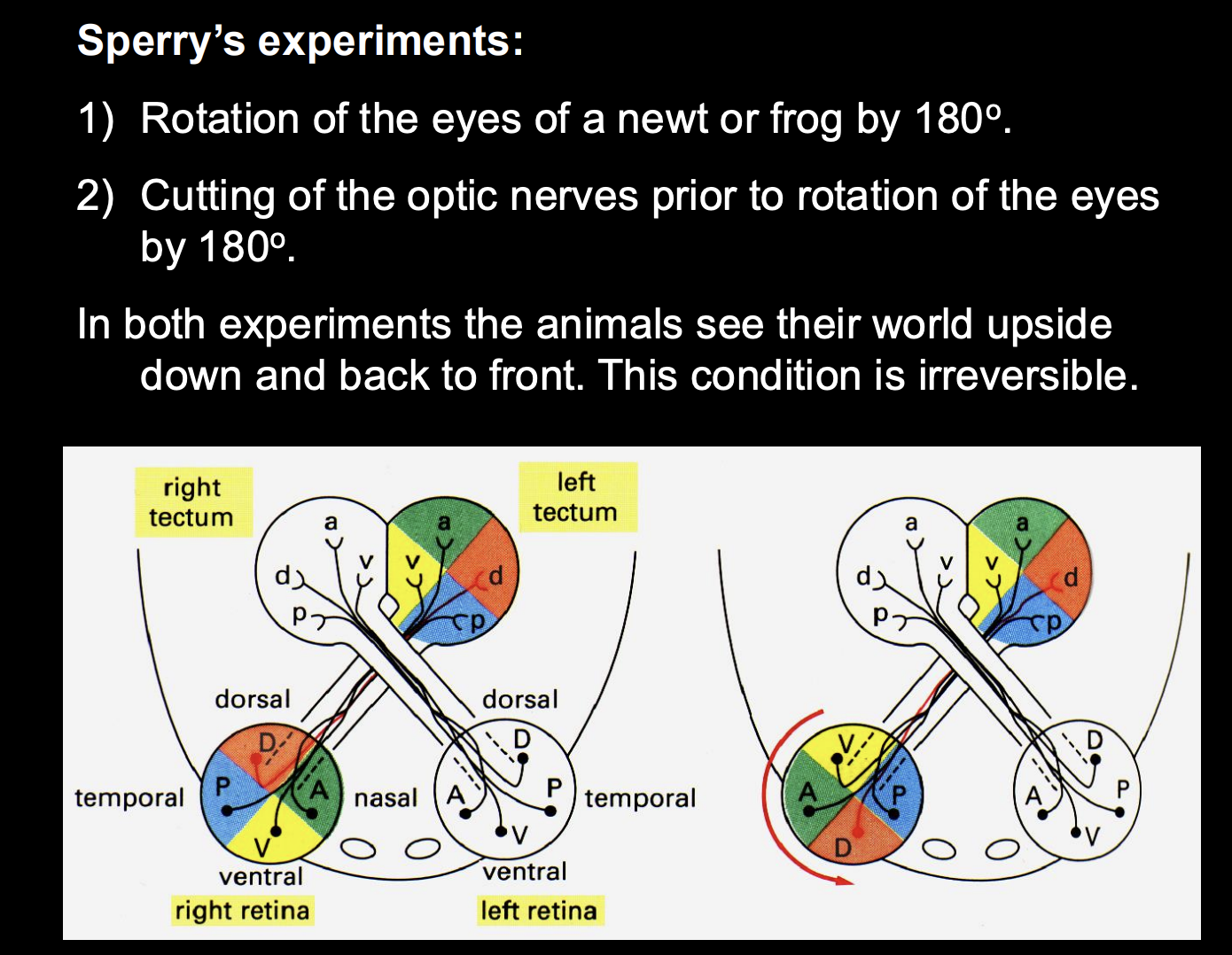
Two conclusions from these experiments:
Precise retinotectal connectivity was not directed by experience→ must be an anatomical feature
retinal axons regenerated after the optic nerve had been cut→ grew back into the tectum and there re-establised synaptic connections at about the same location that they had previously occupied
According to the original anatomical coordinates in the eye
From these conclusions→ Sperry’s chemoaffinity hypothesis
every retinal axons has a particular chemical affinity for a particular location (and thus postsynaptic target) in the tectum
this would explain why there is no functional effect and how it regenerates to the same place as before
because it is under the same influence of chemical affinity stuff
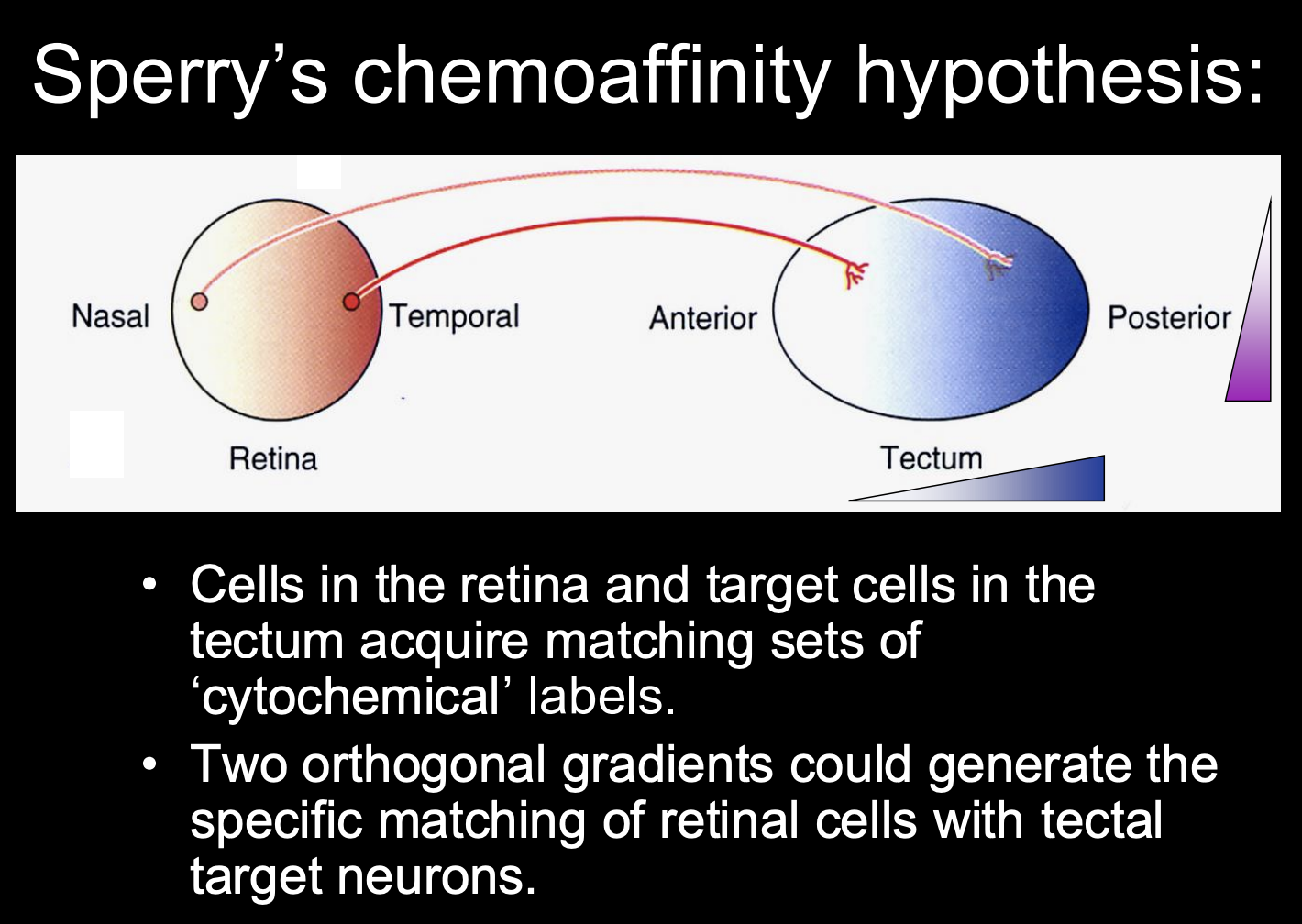

What did Sperry postulate as to how this chemoaffinity hypothesis worked:
a multitude of retinal axons could be mapped onto the optic tectum by two or more perpendicular cytochemical gradients
i.e there are two orthogonal gradients
one in the retina
And one in the tectum
Match up toegther to conserve the retinotopic map as the neurons target onto the tectum from the retina
But what are the mechanisms of this hypothesis?→ next question to ask
how is it that both presynaptic retinal and posynaptic tectal neurons establish their positional identities?
Investigating how these maps are made→ could temporal differences in differentiation and targeting establish the map
Procedure: See when/where the the RGC in the dorsal and ventral arrive in
WT
Retina with dorsal side replaced with another ventral (double ventral)
Results
Wild-type→ Dorsal Root Ganglion cells arrive first
double ventral→ ventral RGC→ select their normal target area
Conclusion:
it is not about the timing of when the RGCs
there is some kind of patterning
it is not just about which part if filled up first
(other wise you should get the WT phenotpye in the double ventral one)
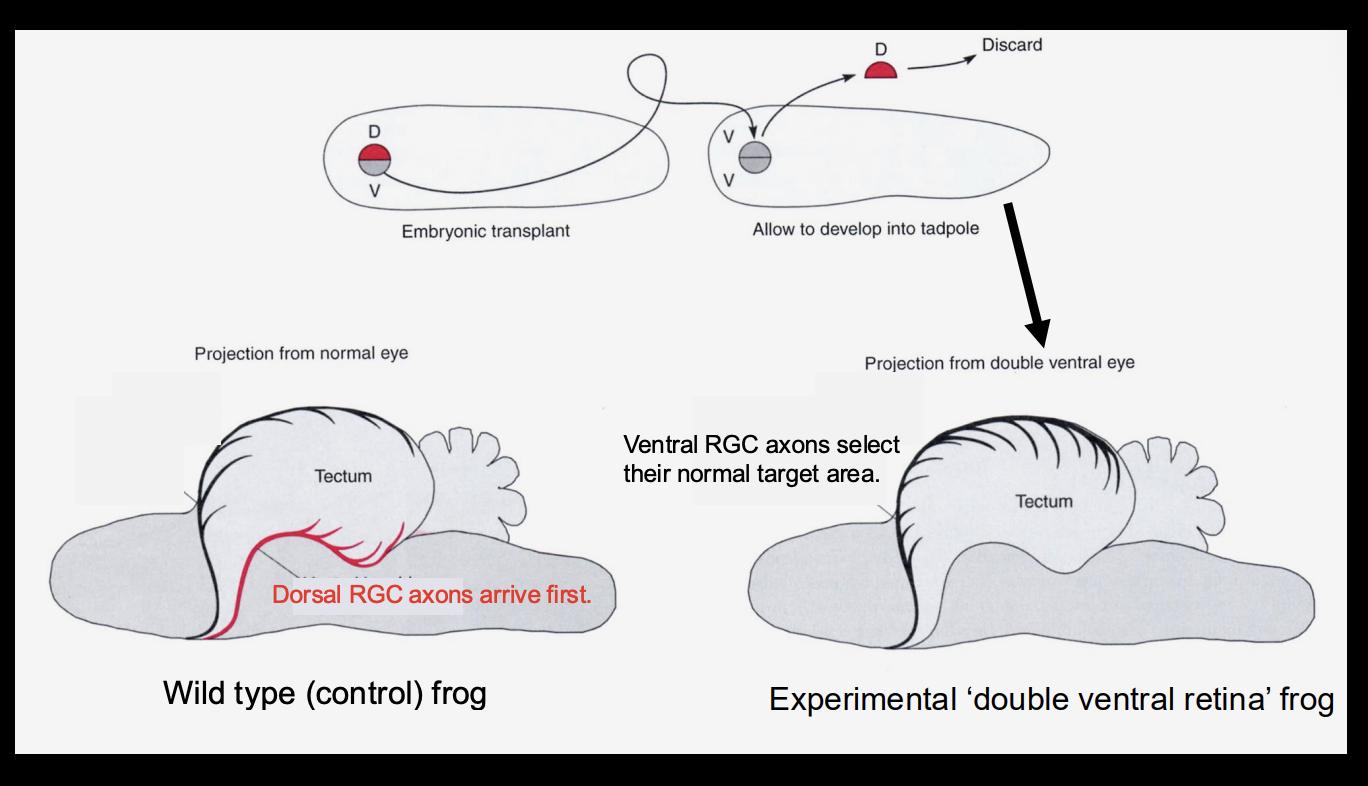
The positional information that confers a retinotopic identity seems to be established when?
during the development of retina and tectum
How Temporo-nasal polarity is set up in the retina (in chicks)
Expression of homeobox transciption factors in non-overlapping domains
BF-1 and BF-2
These confer some positional information to retinal ganglion cells in the Antero-posterior axis
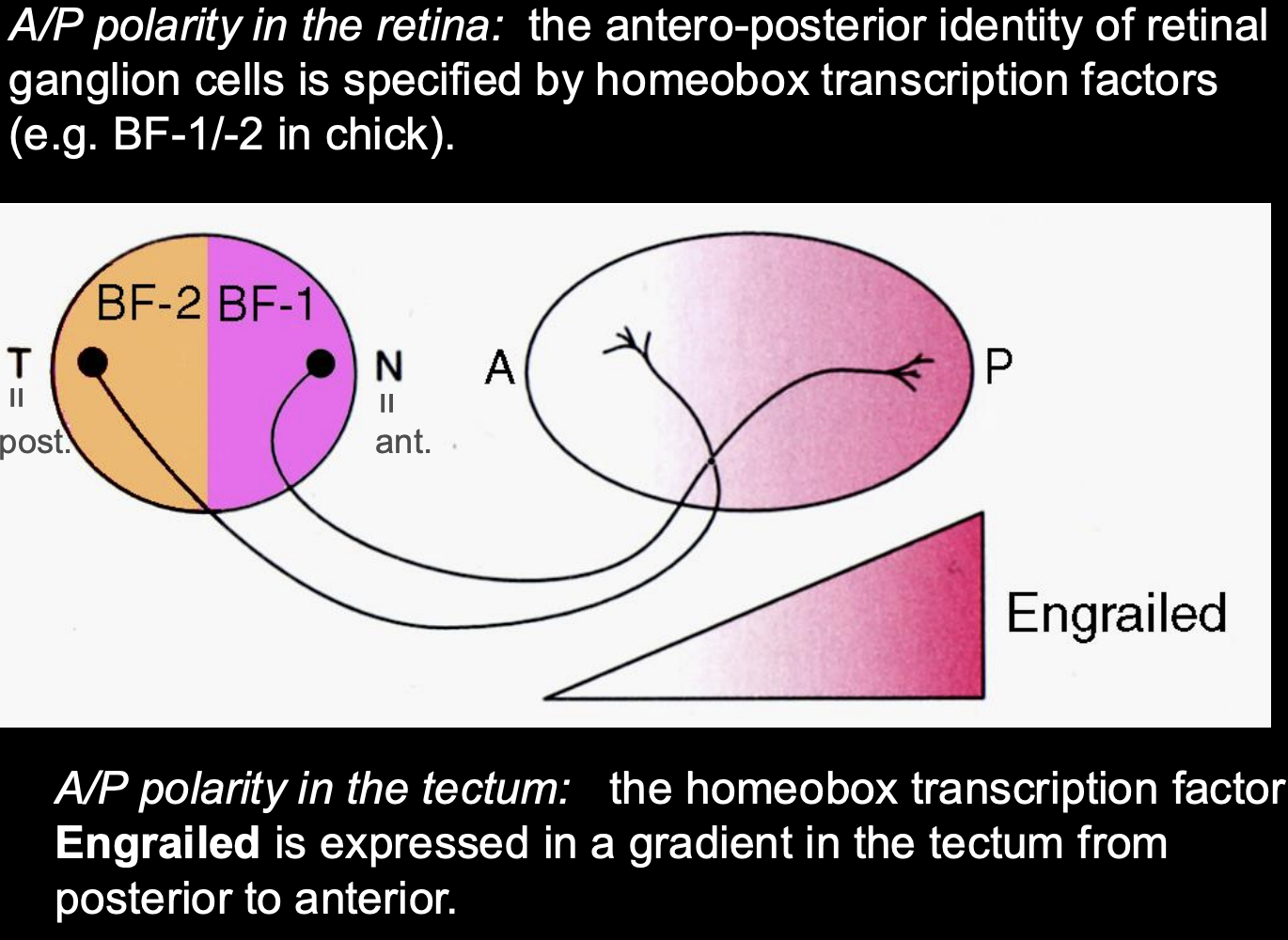
How Antero-posterior polarity is set up in the retina (in chicks)
Gradient expression of Engrailed (En) (works as a transciption factor)
high En→ posterior tectum
low En→ anterior tectum
How was TF engrailed found?
testing RNAs→ cDNA
Find a TF and Apply
see where the axons go (A or P)
found engrailed
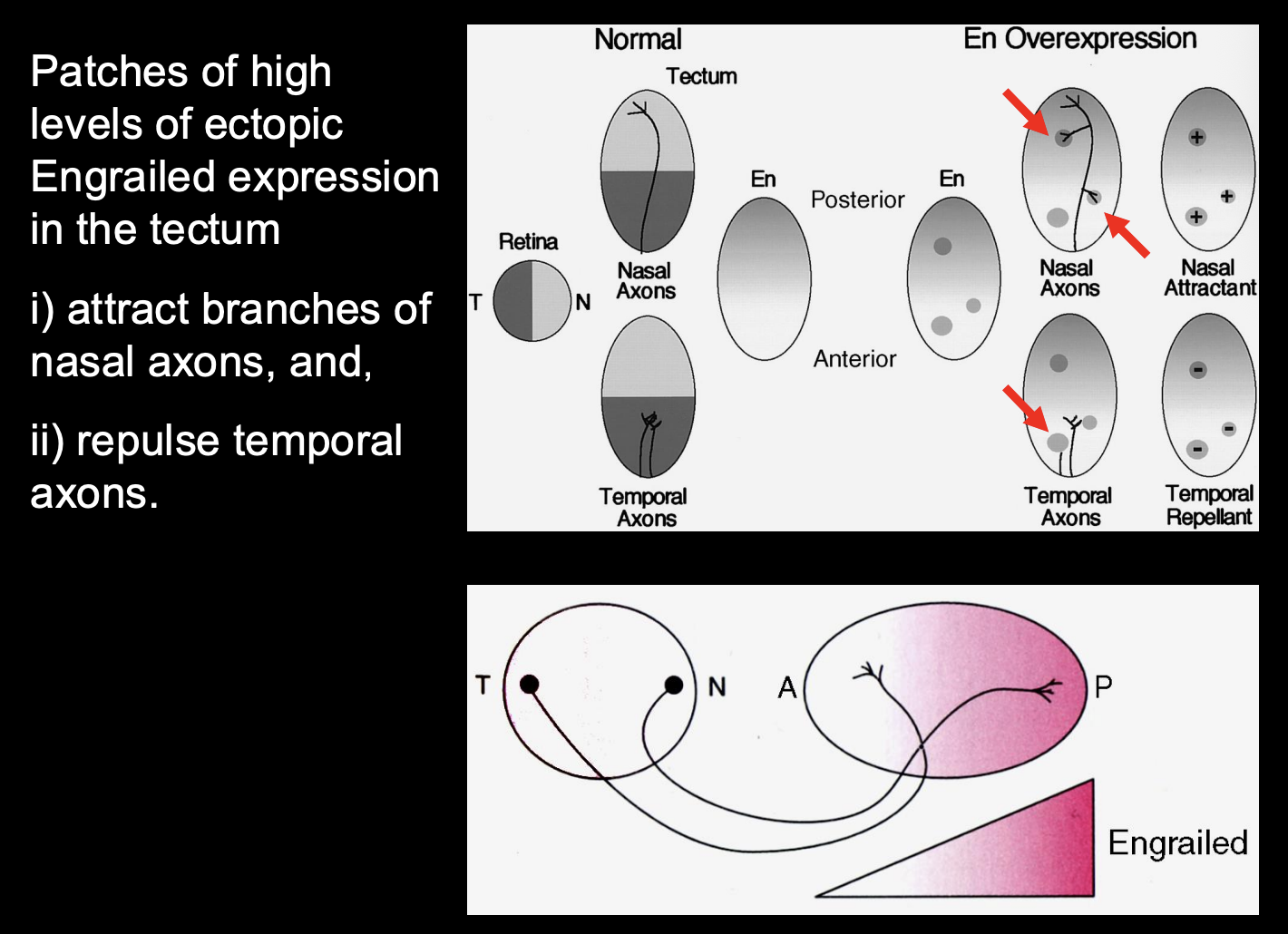
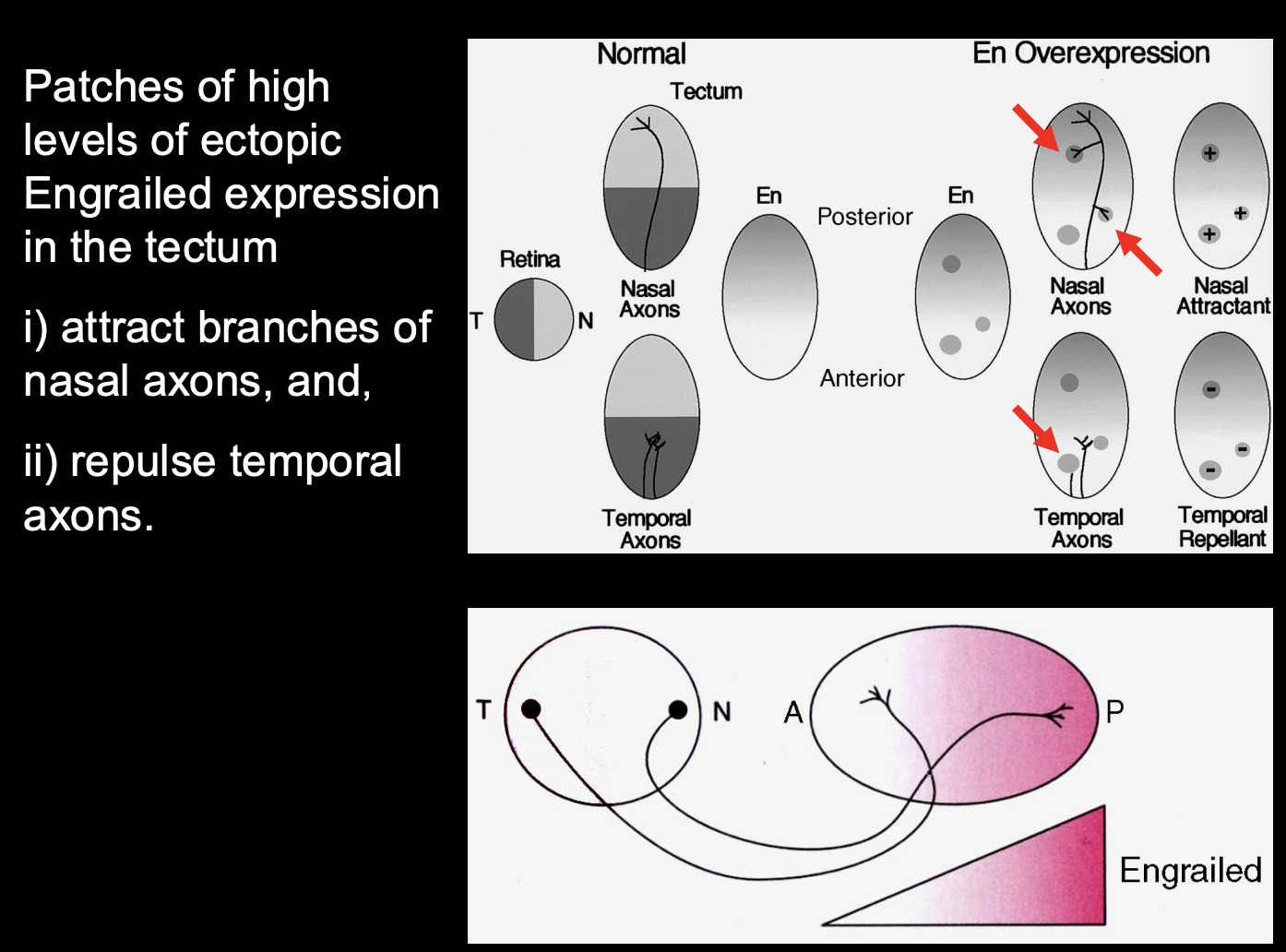
Testing the effect of high and low levels of Engrailed expression
Procedure:
misexpress engrailed using viruses
misexpress in different patches of cells
Result:
High levels of ectopic Engrained expression =
Attract branches of Nasal axons
Repulse temporal axons
Therefore→ different ganglion cell axons from different places in the retina have a different response to high levels of engrained
engrailed must be regulating guidance molecules for retinotopic mapping
How were guidance molecules used for retinotopic mapping (that engrailed might be regulating) investigated
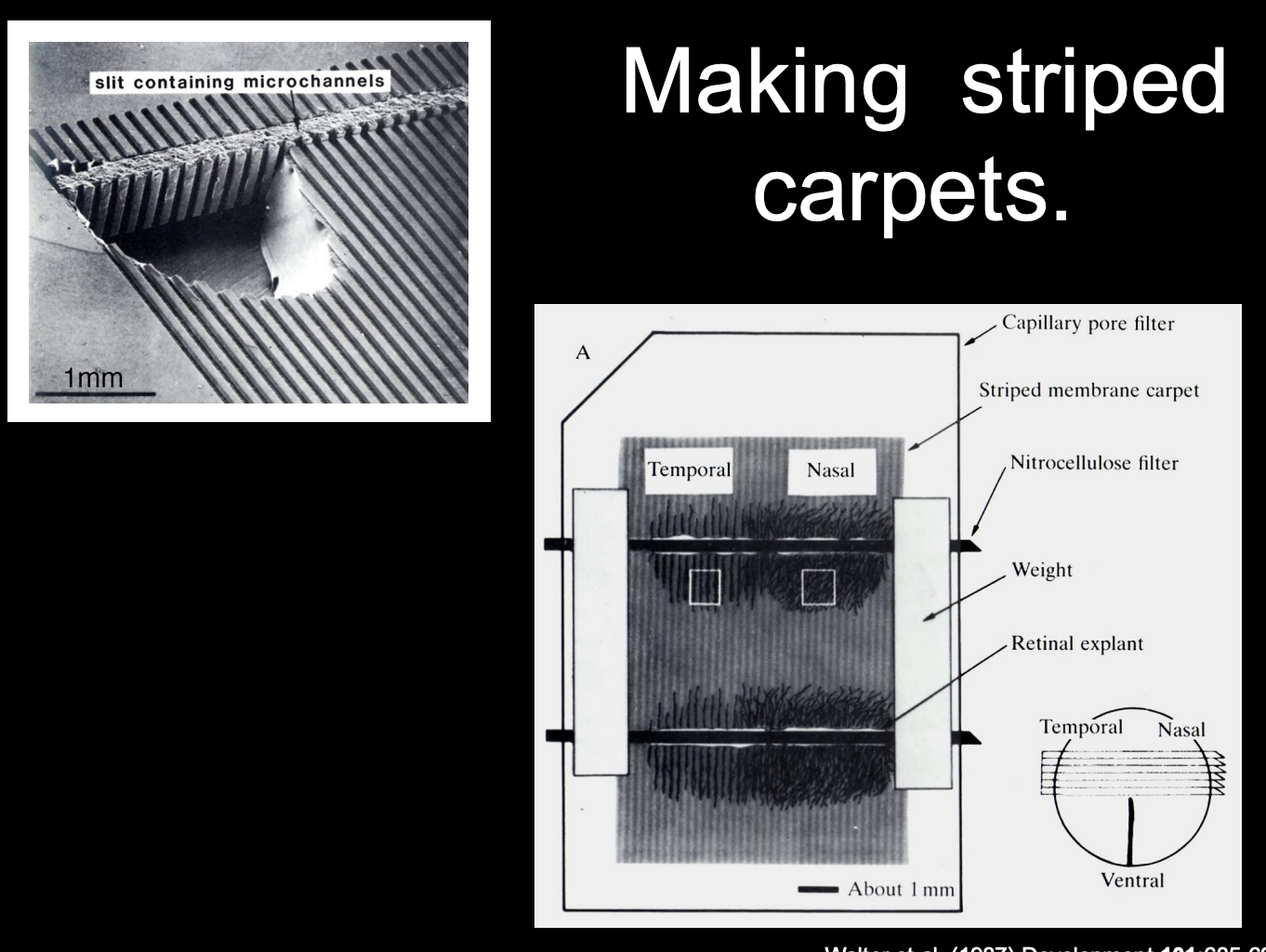
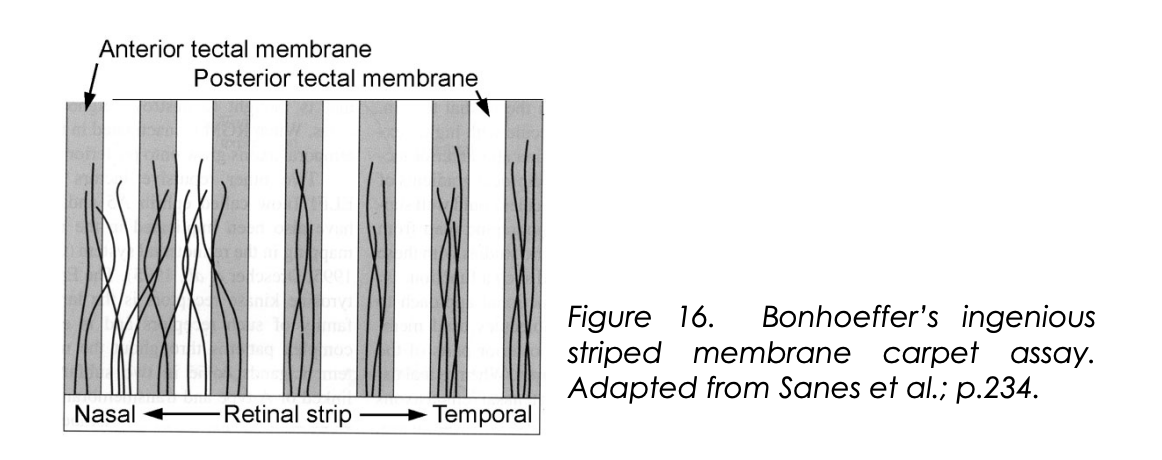
Bonhoeffer
→ Microscopic carpets→ striped carpet assay
alternating stripes of membrane derived from either anterior or posterior parts of the tectum
How are these striped carpet assays made
put nasal or temporal axons in stripes
onto anteror and posterior regions of the tectum
See where they want to go
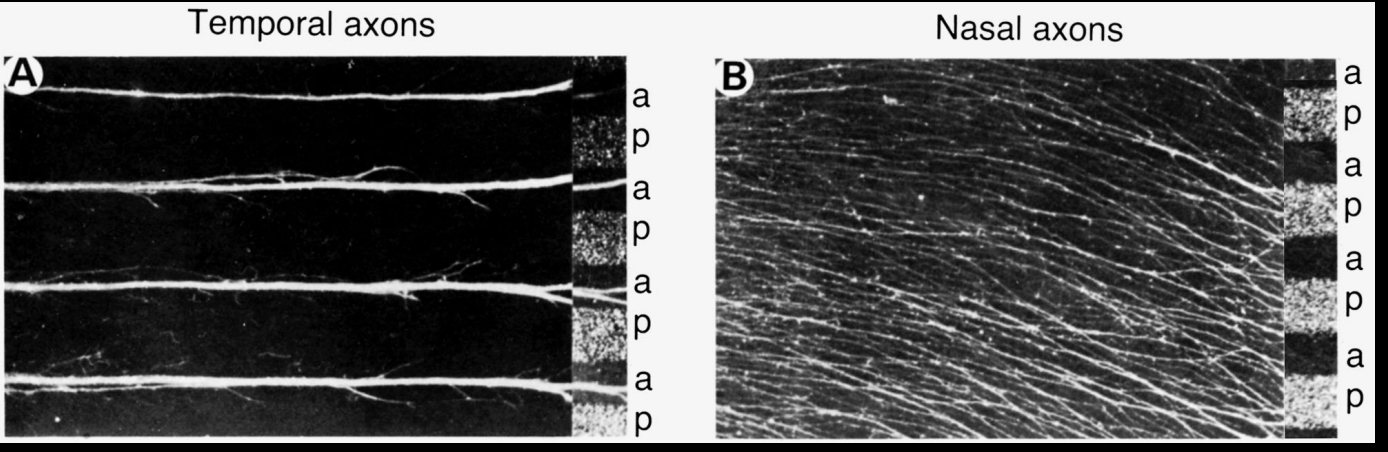
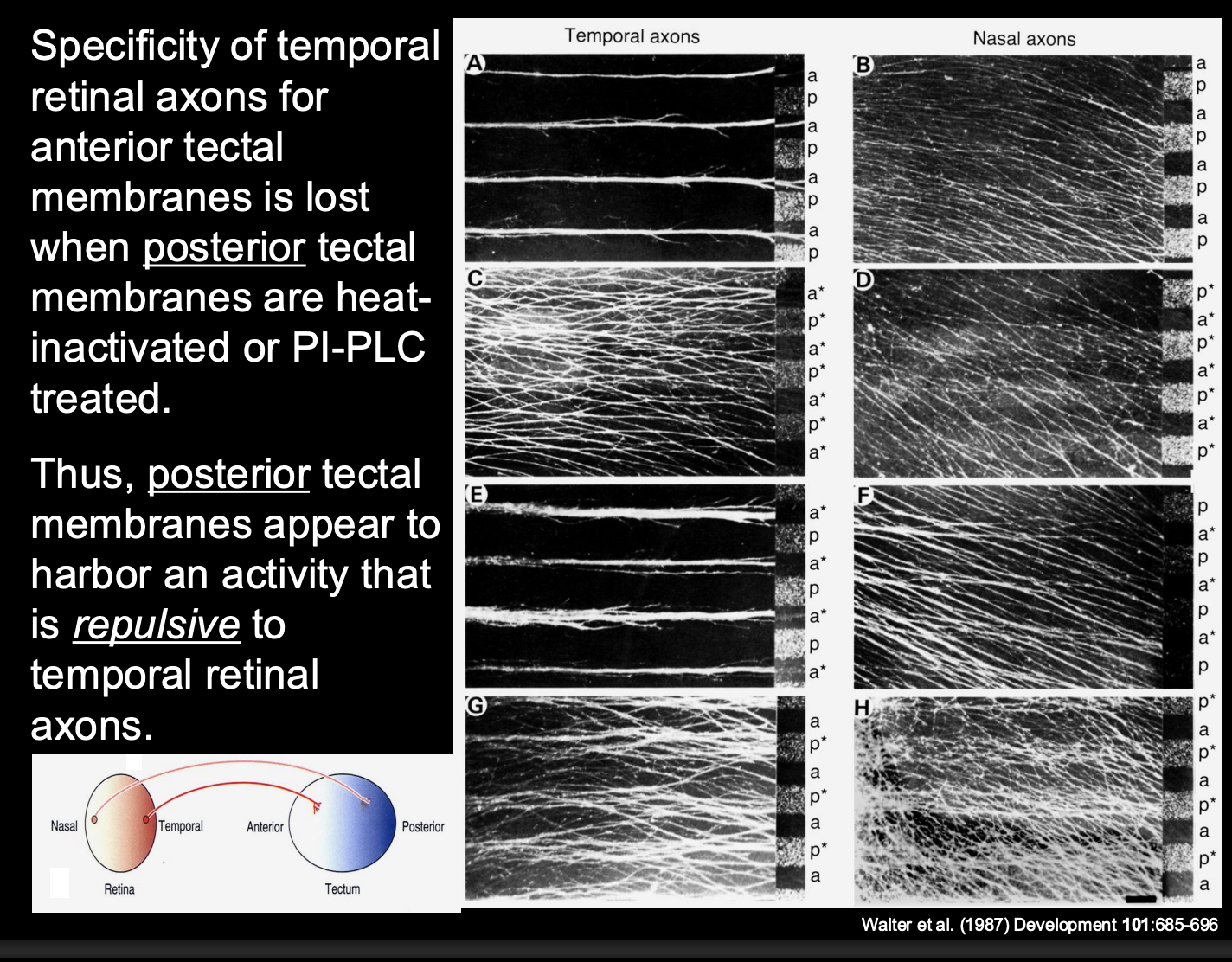
Results from the striped carpet assay
Temporal retinal axons→ prefer Anterior temporal membranes
Nasal retinal axons→ no preference
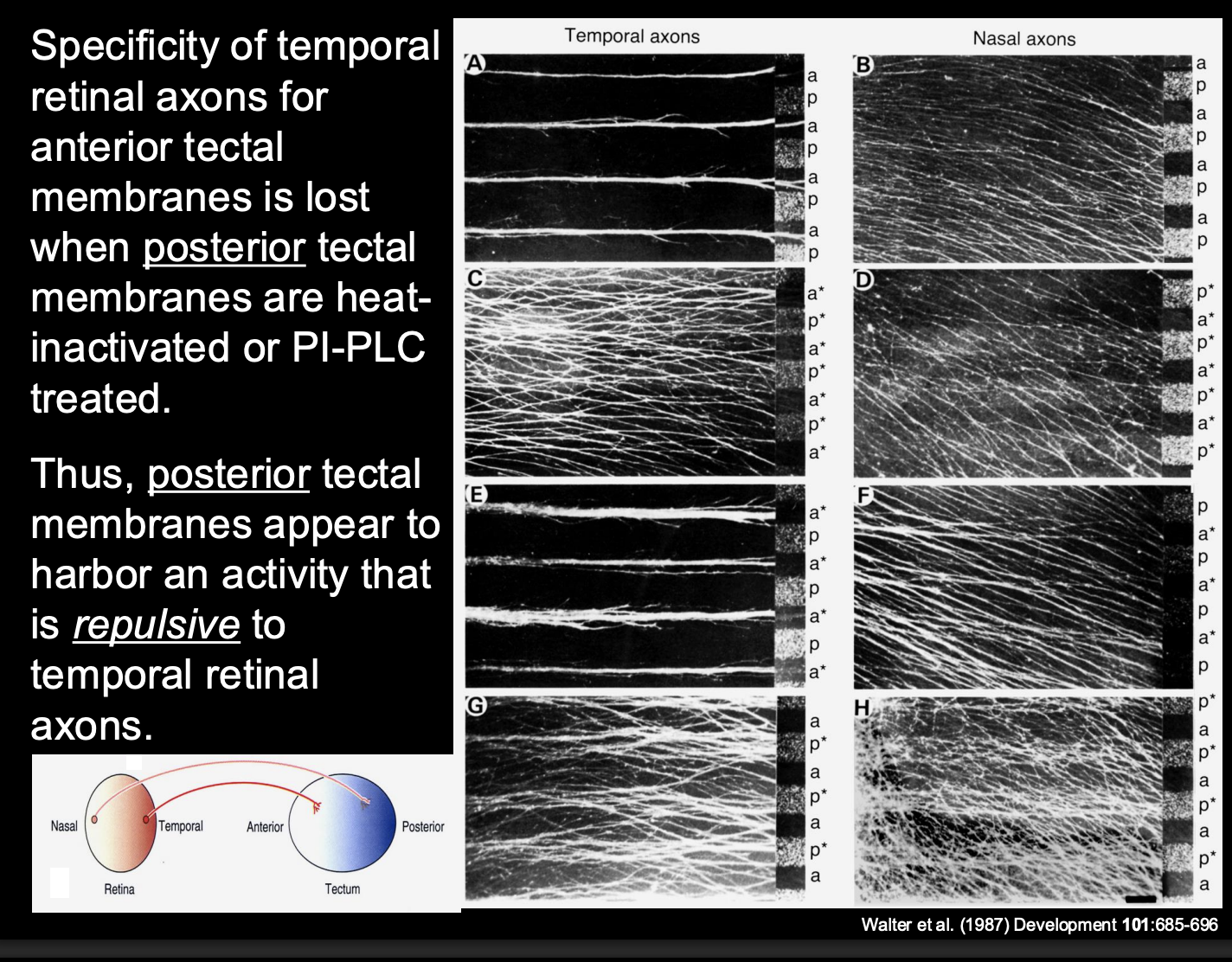
Investigating further→ finding the biochemical components that restrict the temporal retinal axons to the anterior only
Procedure:
heat treat specific membranes
or→ incubate with an enzyme (PI-PLC)
destroys phosphotidyl-inositol (PI)linked membrane molecules
Result:
If disrupt posterior tectal membranes—> Temporal axons now also go to the posterior
loss of preference for the anterior
conclusion:
There is repulsion from the posterior tectal on the temporal axons
what was this repulsive function found to be?
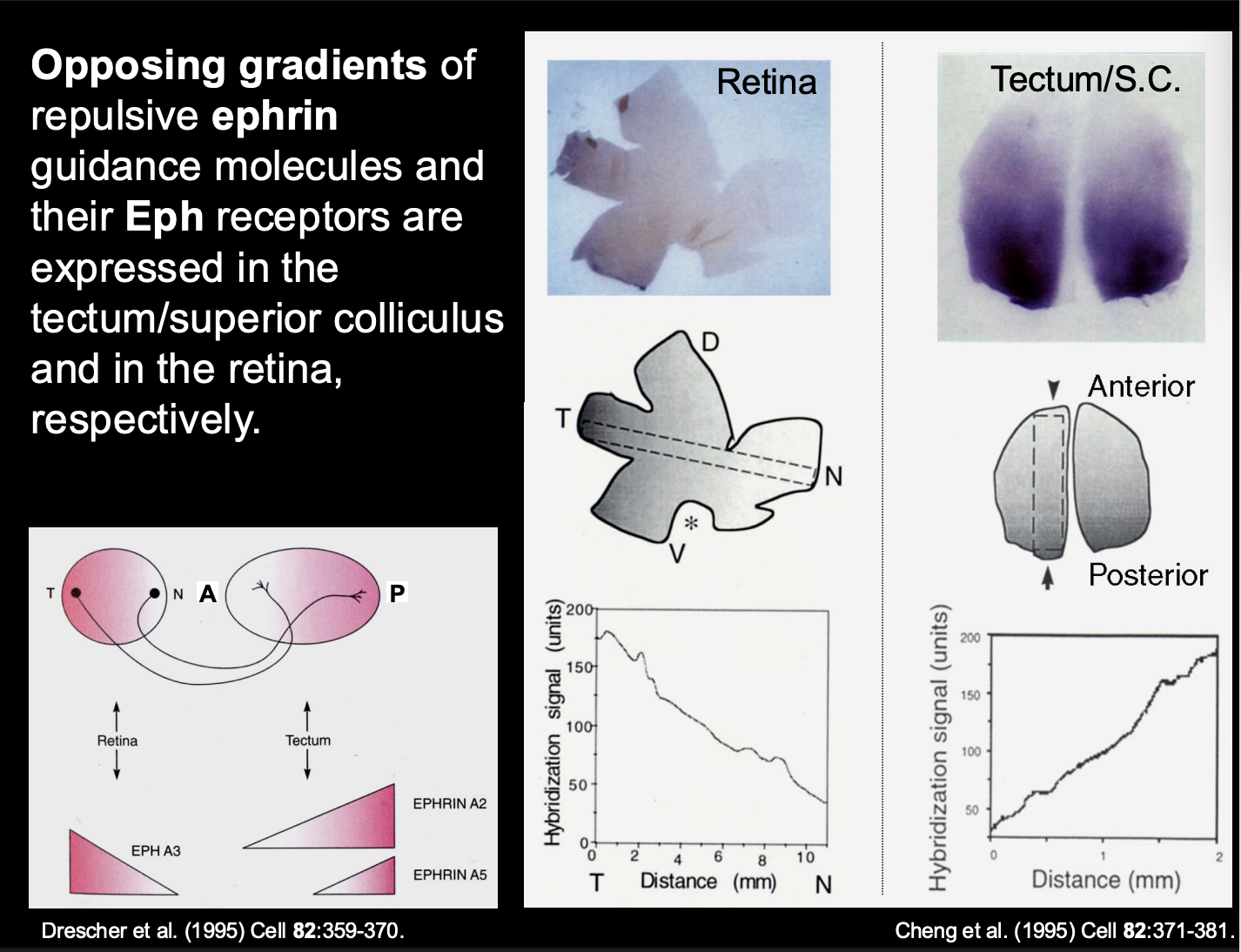
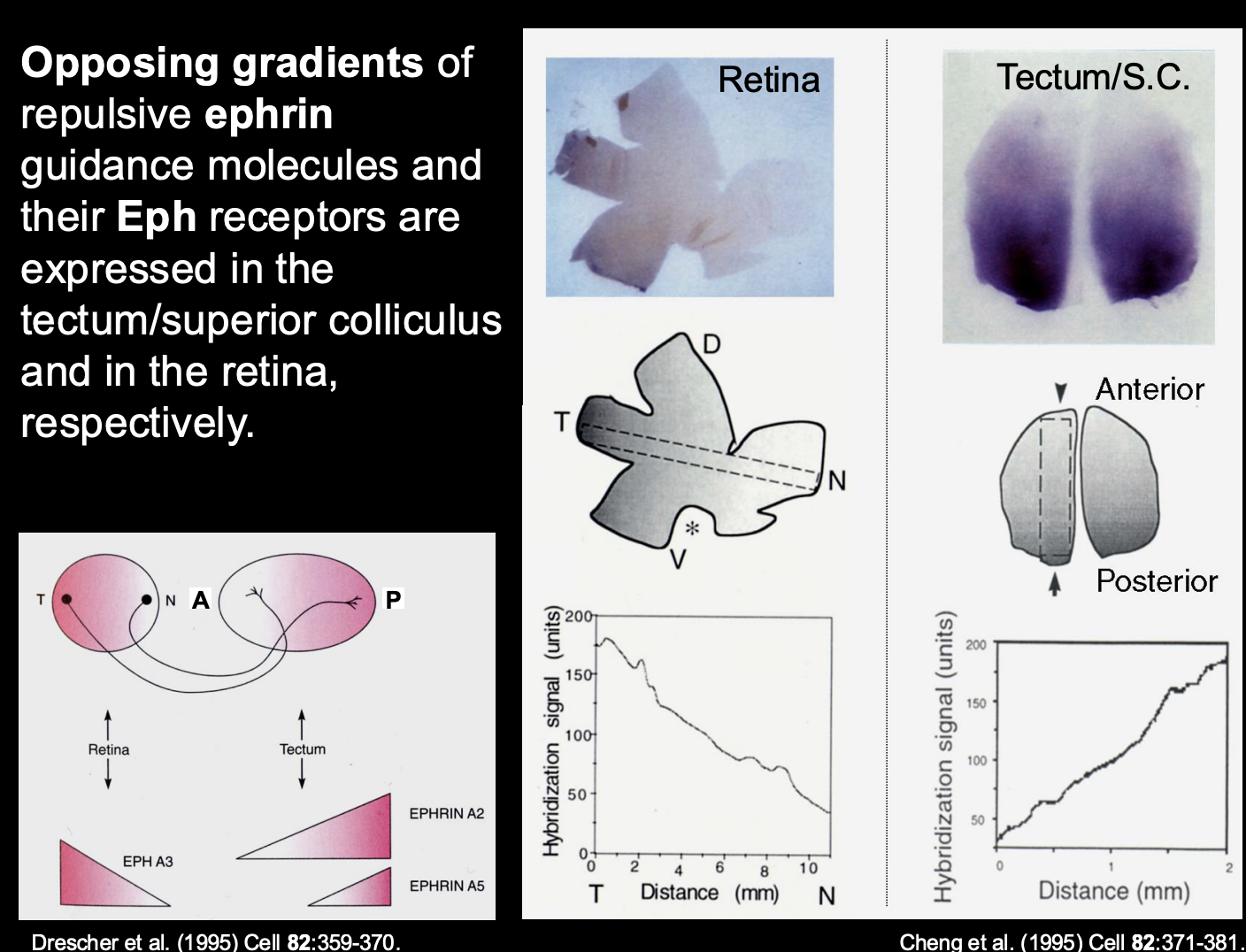
mediated by a member of the family of Ephrin (Eph) ligands
these set up biochemical gradients
What kind of molecules Eph ligands
A-type→ GPI-linked molecules
B-type→ Transmembrane molecules
Receptors for these ligands
Have a large family of receptor tyrosine kinases
Correspond to A or B types
Therefore
All A-type Ephrin ligands→ activate all A-tpye Eph receptors
All B-type Ephrin ligands→ activate any B-type Eph Receptor
i.e specific ligands go to specific recetprso (similar to what is seen in motor neuron?)
If all e.g type A can interact with type B→ how do you get a wide scope of signalling?
Ligand-receptor pairing have differential affinities for one another
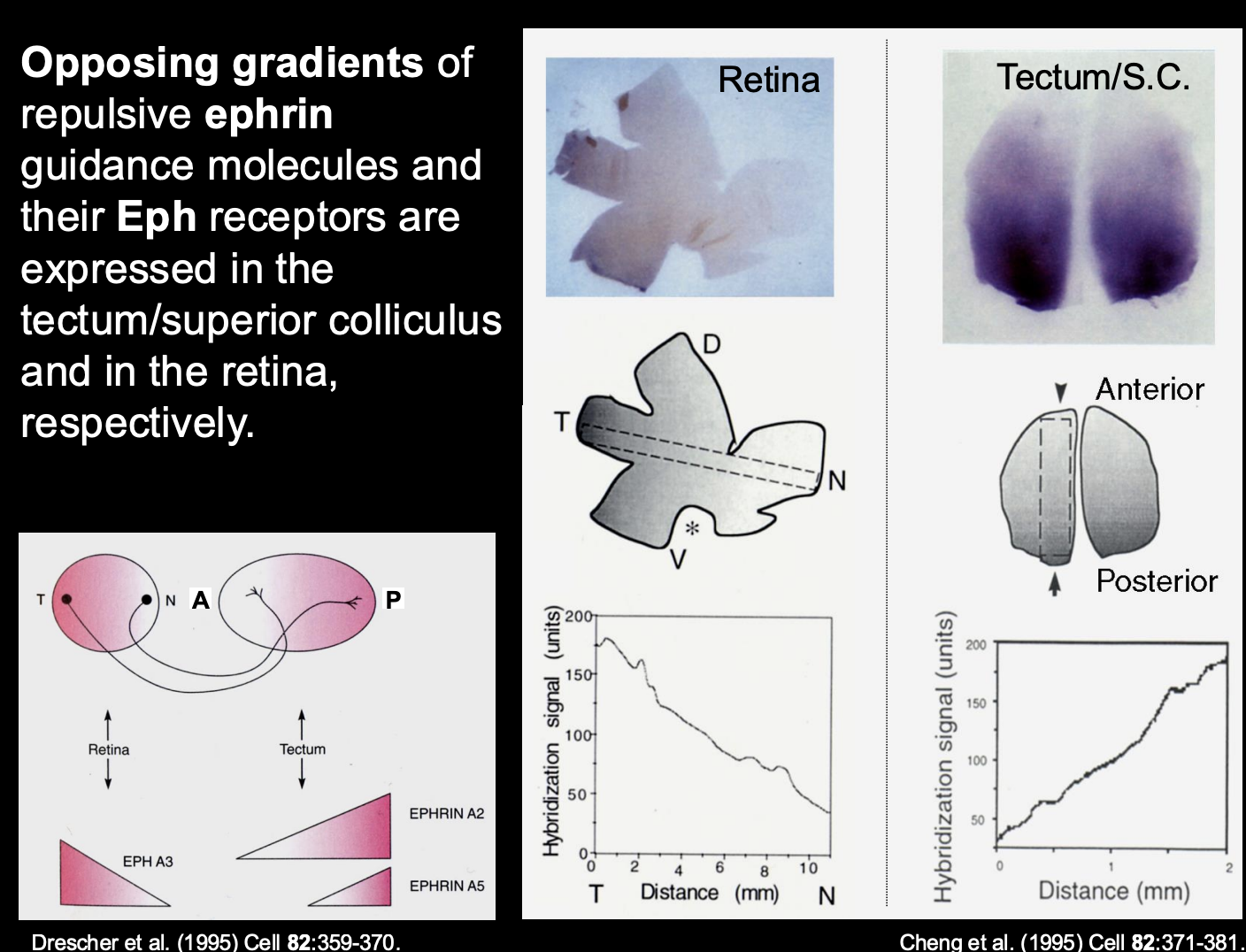
What are the biochemical gradients of ephrin in the tectum
A2 and A5
High levels → Posterior
Low levels → Anterior
i.e must be the repulsive force for getting temporal axons to the anterior
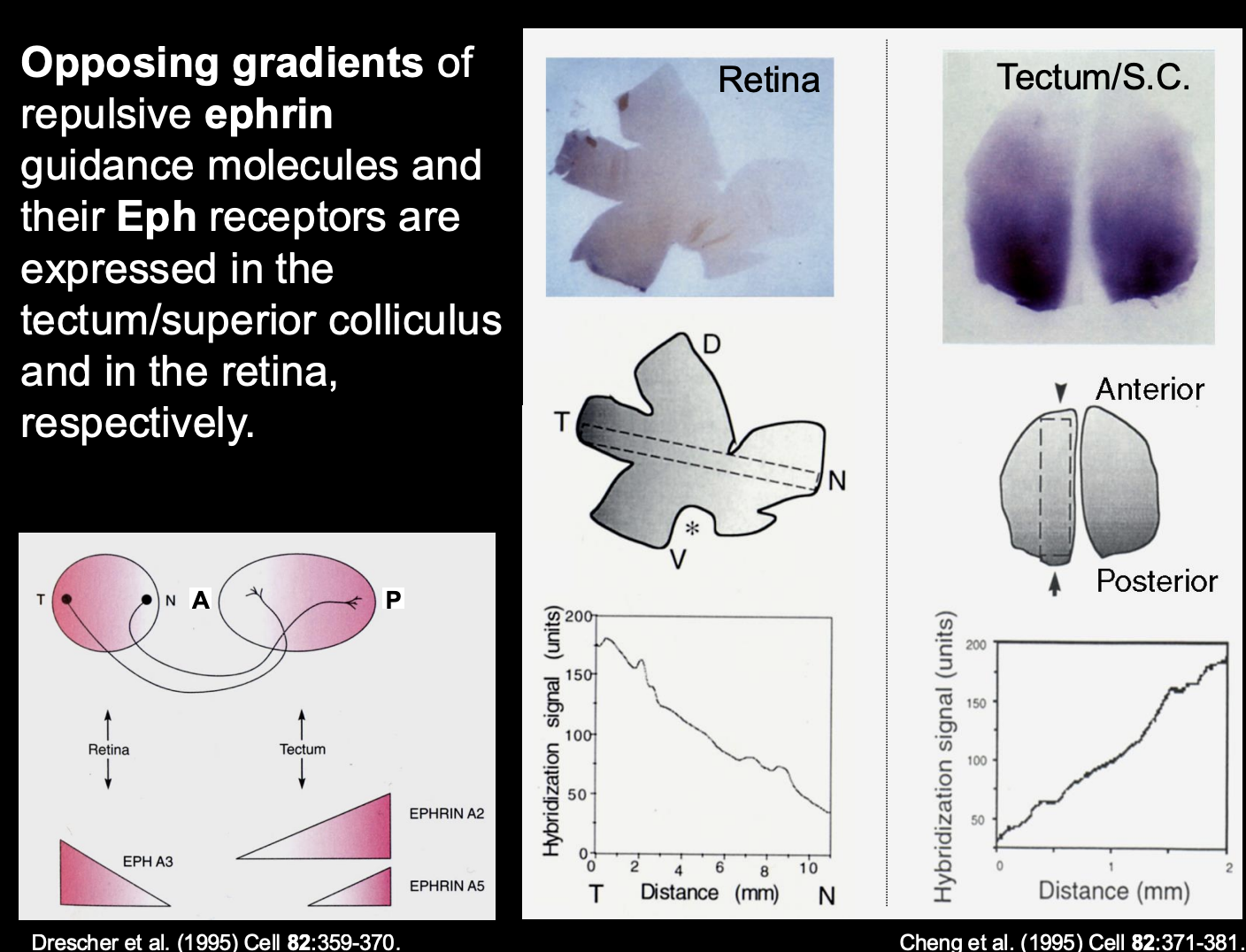
Therefore what is the retinal axon receptor pairing to the tectum biochemical gradient (i.e how is temporal retinal ganglion to the posterior)
Temporal retinal ganglion cells→ Express high levels of EphA3 receptor
receptor for the high levels of the A2 and A5 in the posterior tectum
Causes→ repulsion of the posterior
Further evidence that ephrin A2 repels the temporal axons:
when ephrin-A2 is virally over-expressed at high levels throughout the tectum
→ temporal retinal axons fail to make terminal projections into the tectum
When under-expressed (ephrin-A5 knockout)
→ ectopic arborisations of termporal retinal axons in the posterior part of the tectum
i.e no longer being repelled from the posterior
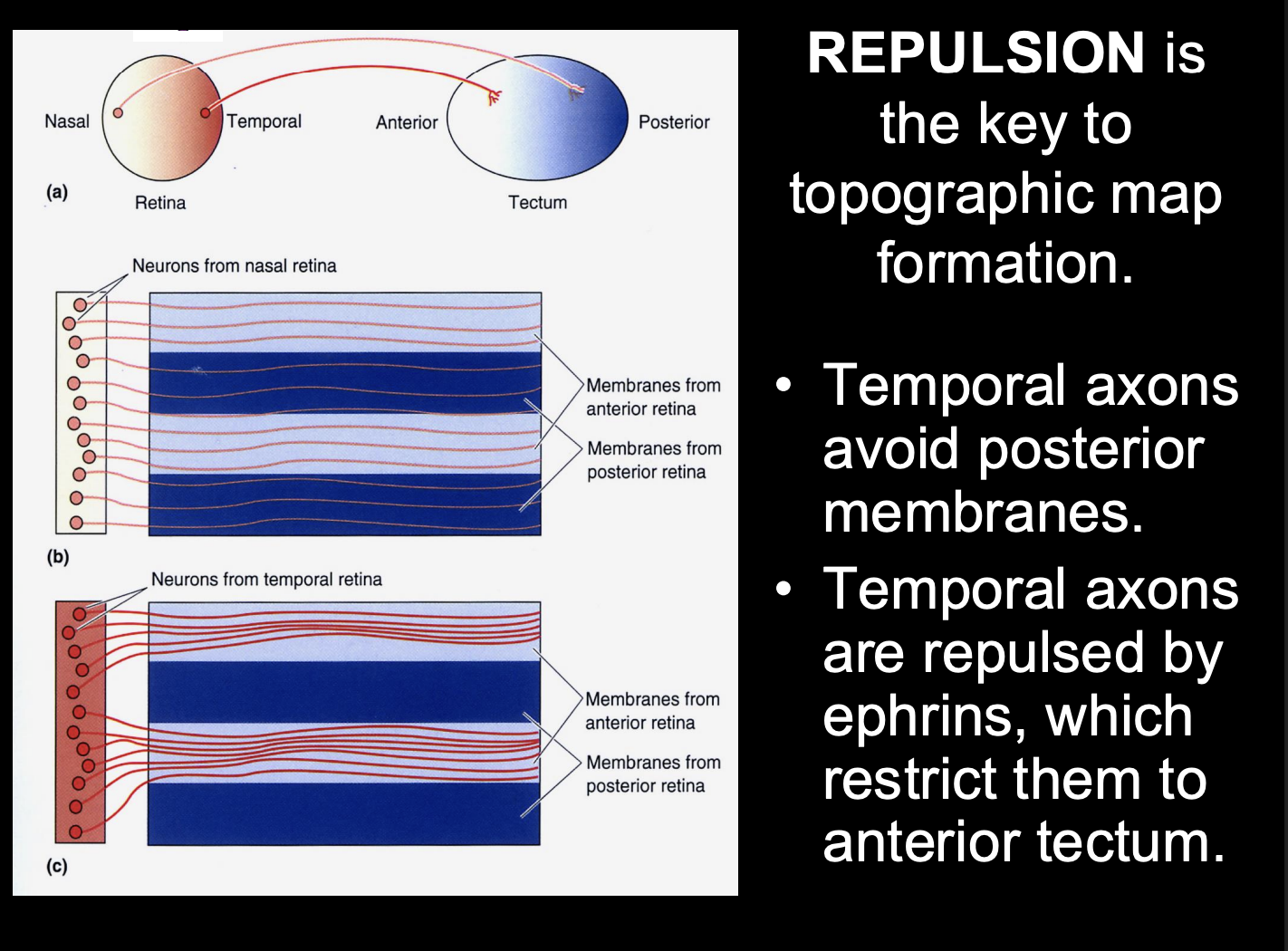
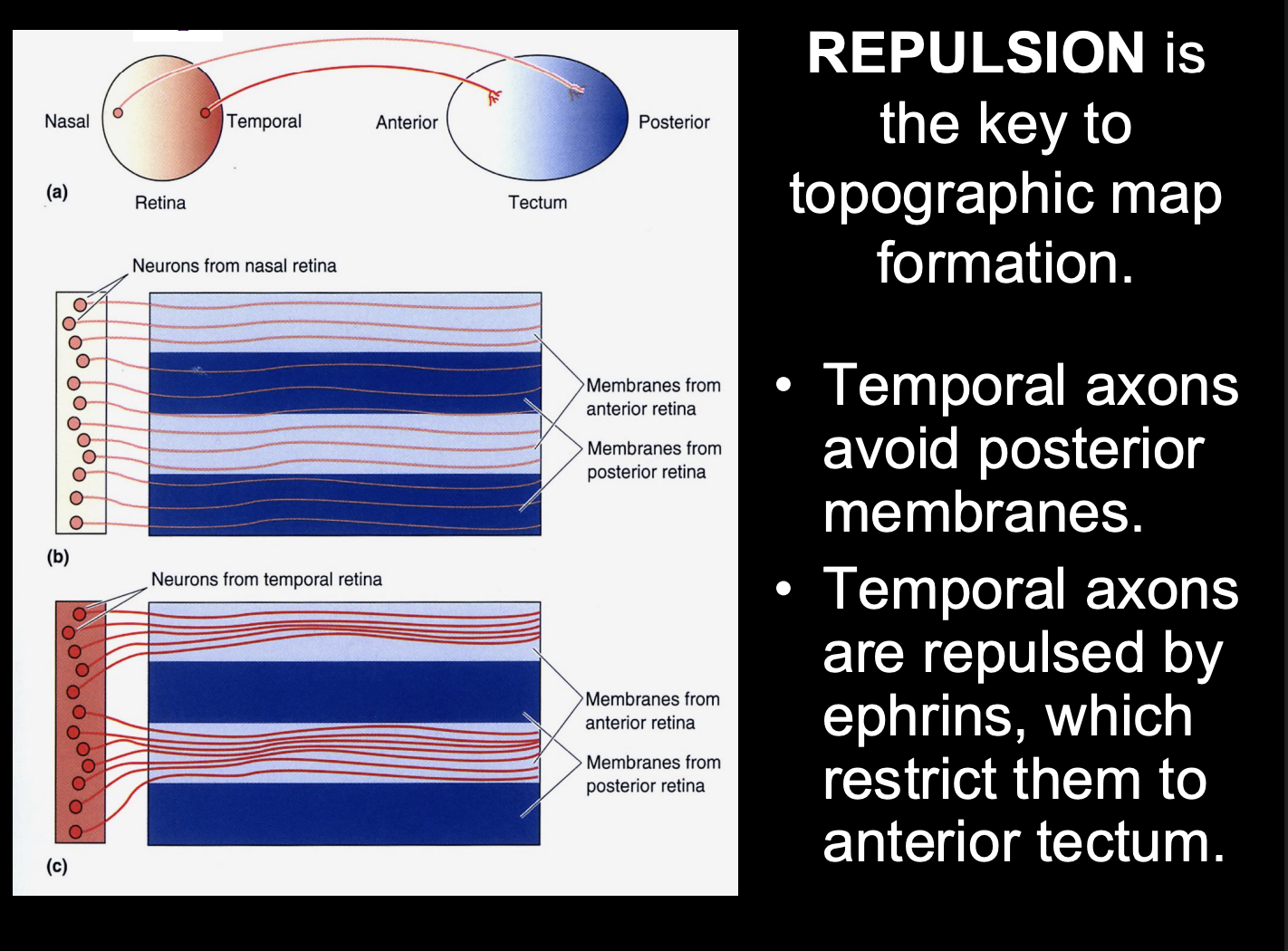
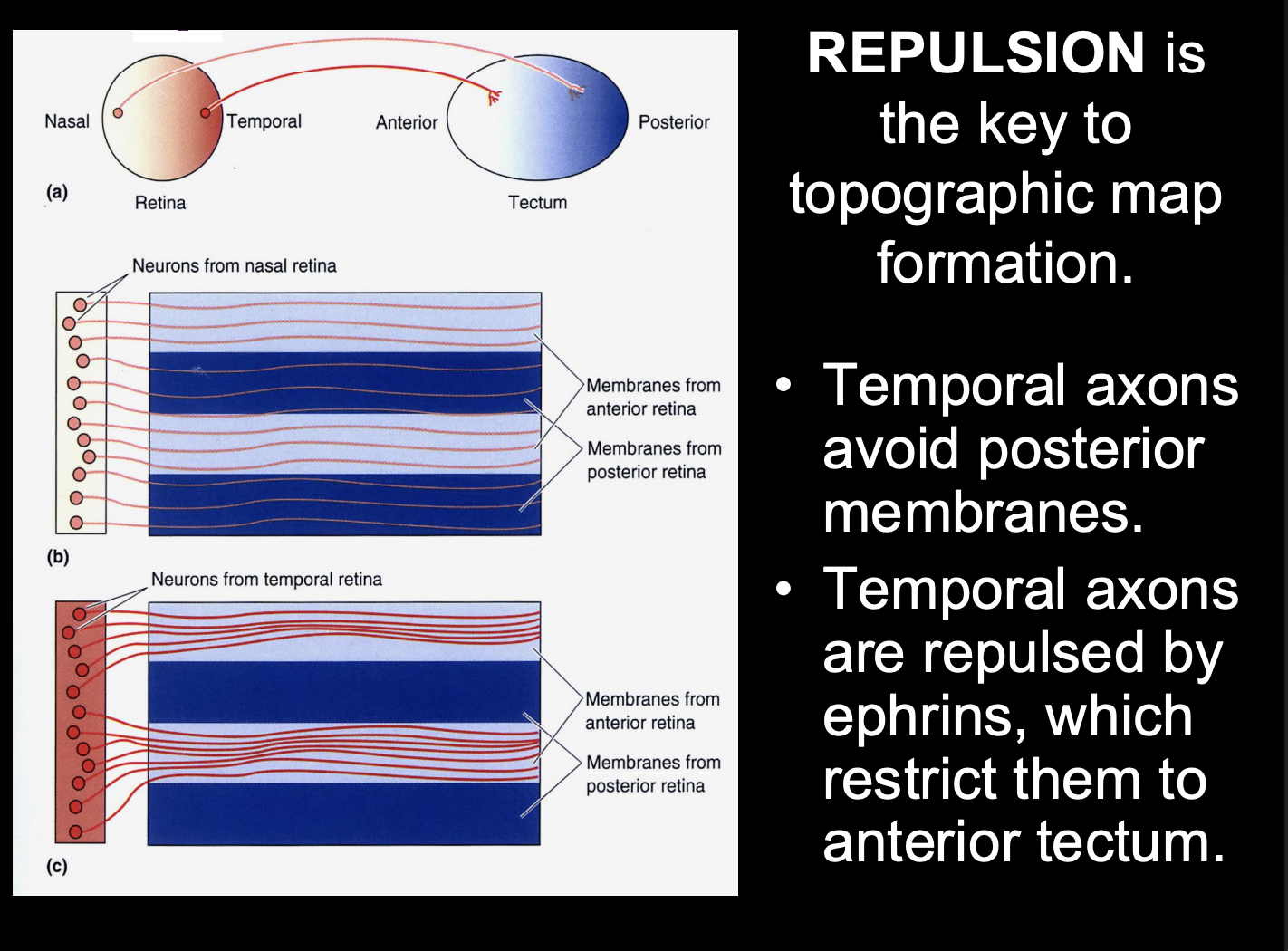
Overall this shows us that
repulsion is key to topographic map formation
but this only explains the temporal axon pathway
How are these gradients of ephrin A2 and A5 expressed?
Due to the expression profile of En (Engrailed)
Indeed this has been proven
therefore shows how the TF is setting up these gradients
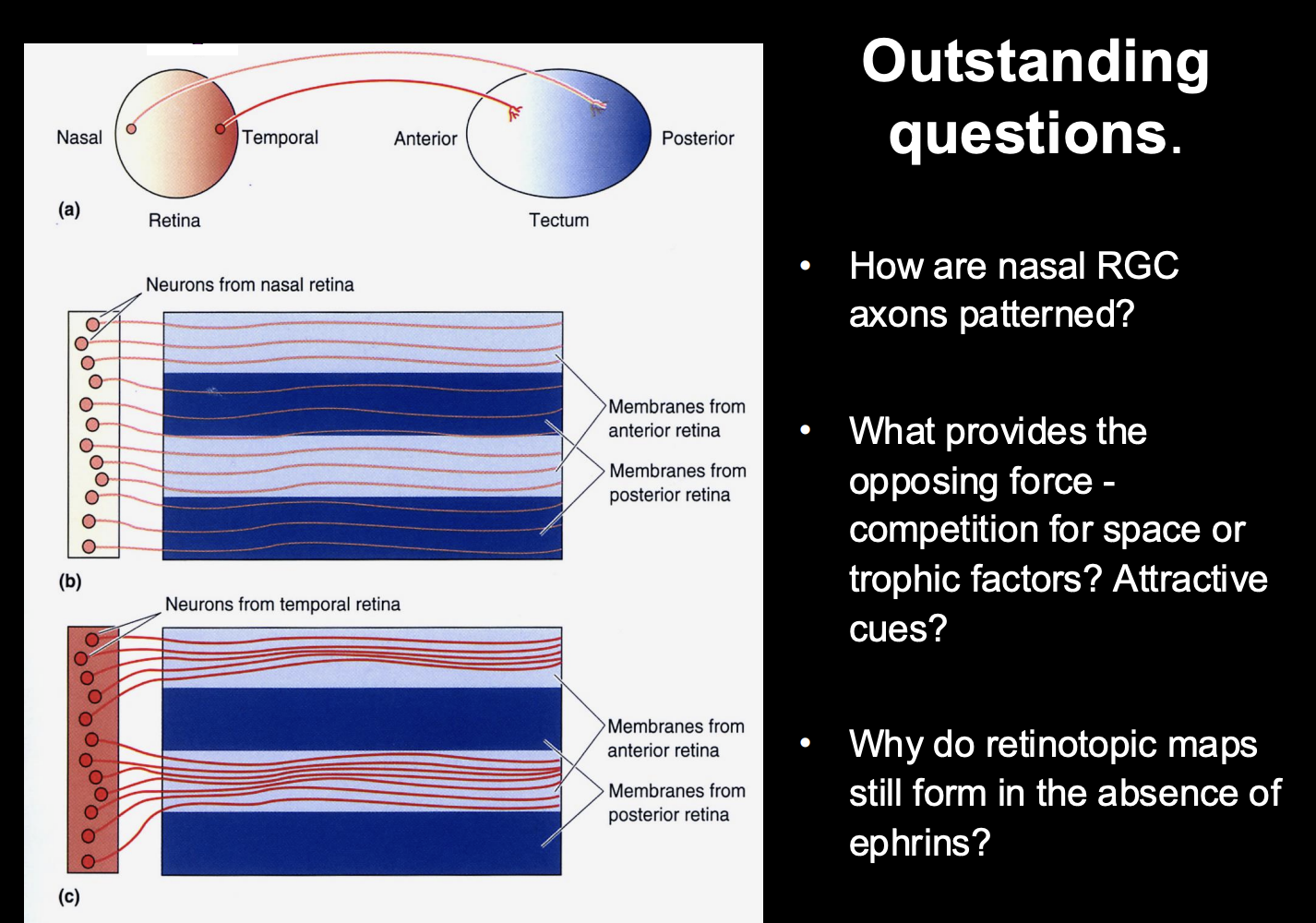
But questions that have not been answered
How are nasal RGC axons patterned?
computational models predict a requirment for at least two opposing forces for topographic mapping
What actually provides the opposing force?
competition for space or trophic factors?
Attractive cues?
Why do retinotopic maps still form in the absence of ephrins??
Repulsion does not cause precision→ how does it get so prescise?
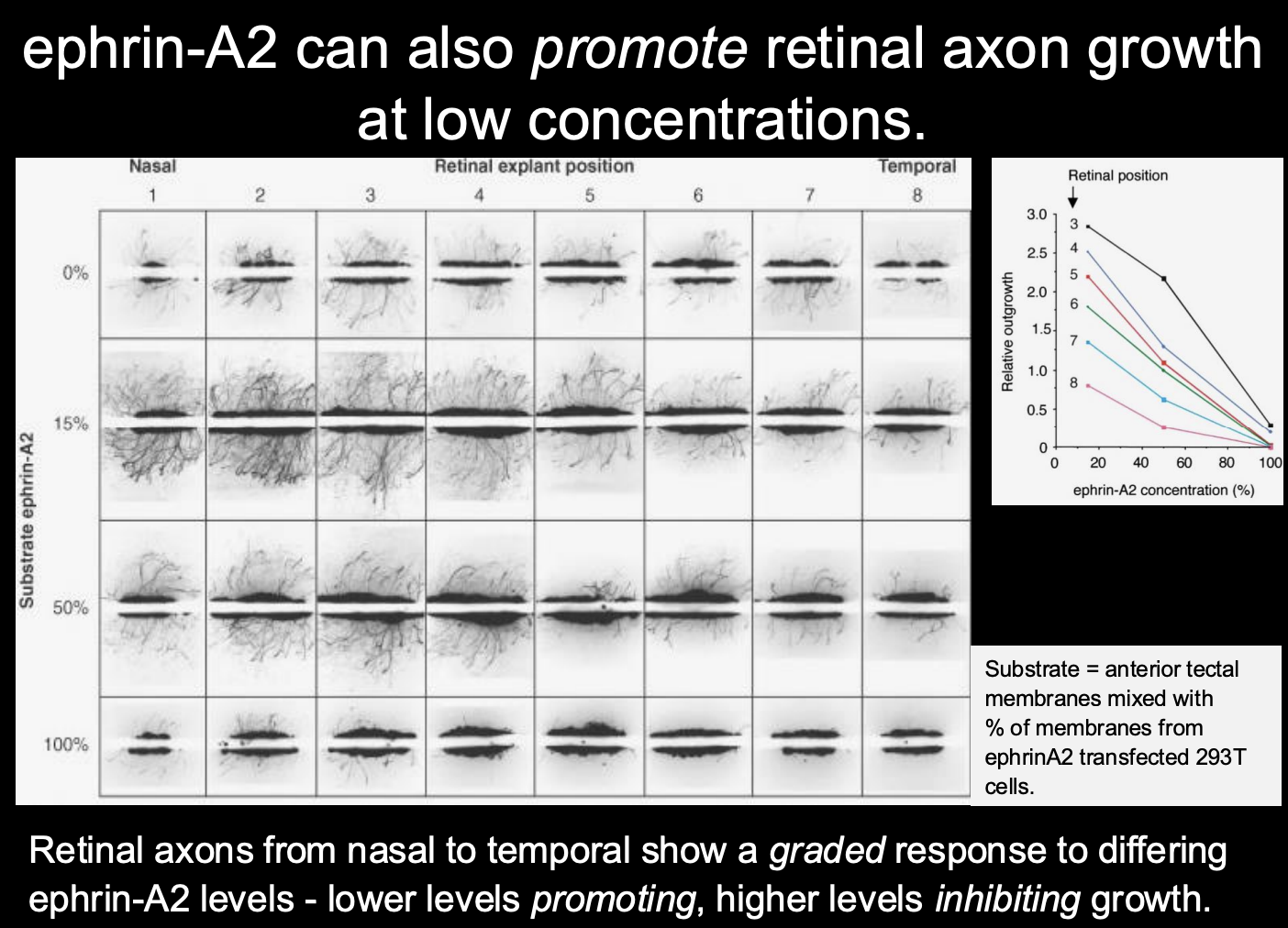
How are the retinotectal projections of the nasal axons patterned?
EphA-ephrinA interactions actullay have different effecst at different concentrations
higher concentration→ repulsion (for temporal)
lower concenctration→ prmote retinal axon growth
therefore shows: dual concnetation dependent response
guidance cues can mediate both forward and reverse signalling
therefore: this signalling system can provide the requirred two opposing gradients through
differing receptor responces from differeing concentrations
overall→ can get temporal and nasal mapping from the same gradients!
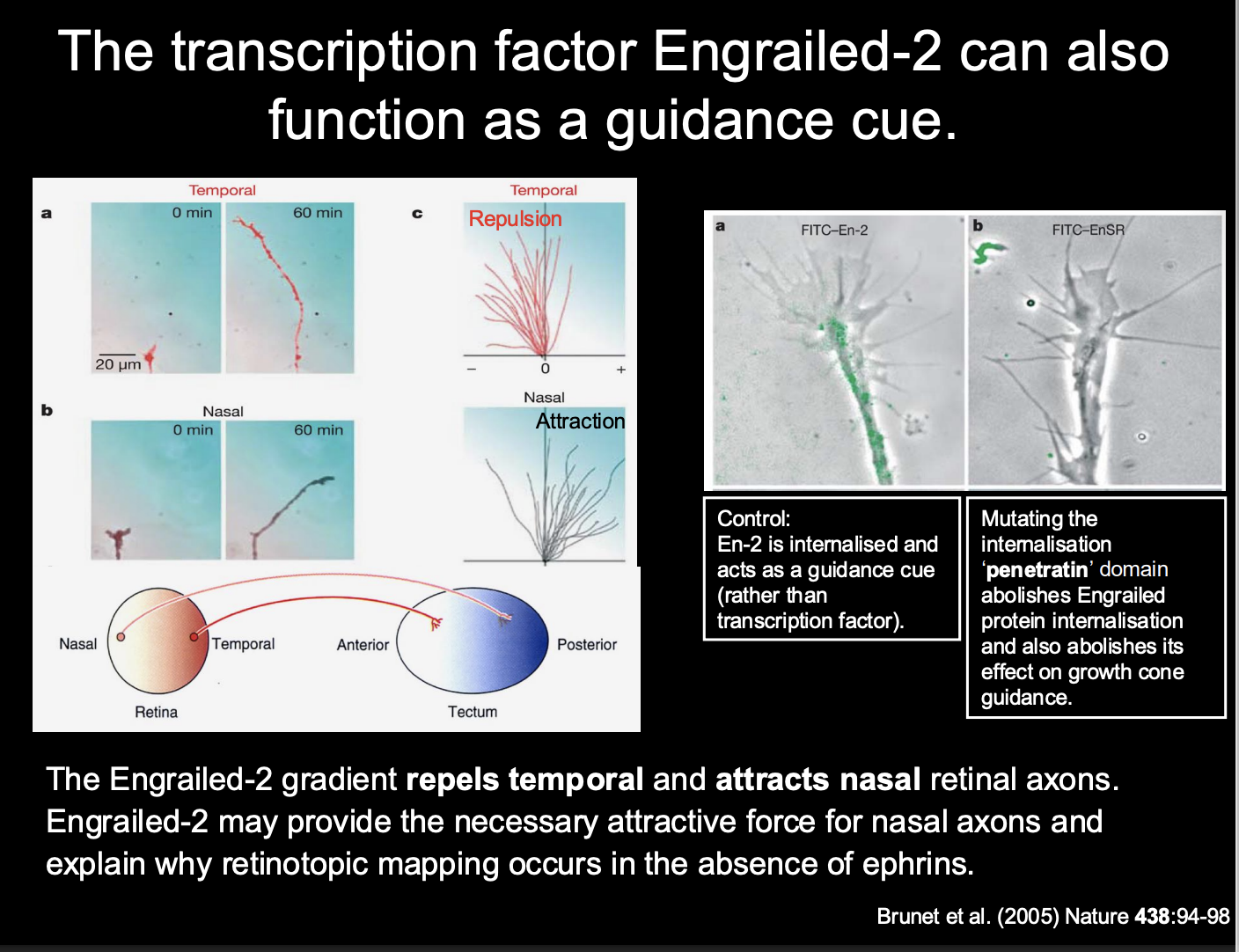
Why do retinotopic maps still form in the absence of ephrins??
other signalling molecules are present in the developing visual system
Example→ TF Engrailed itself
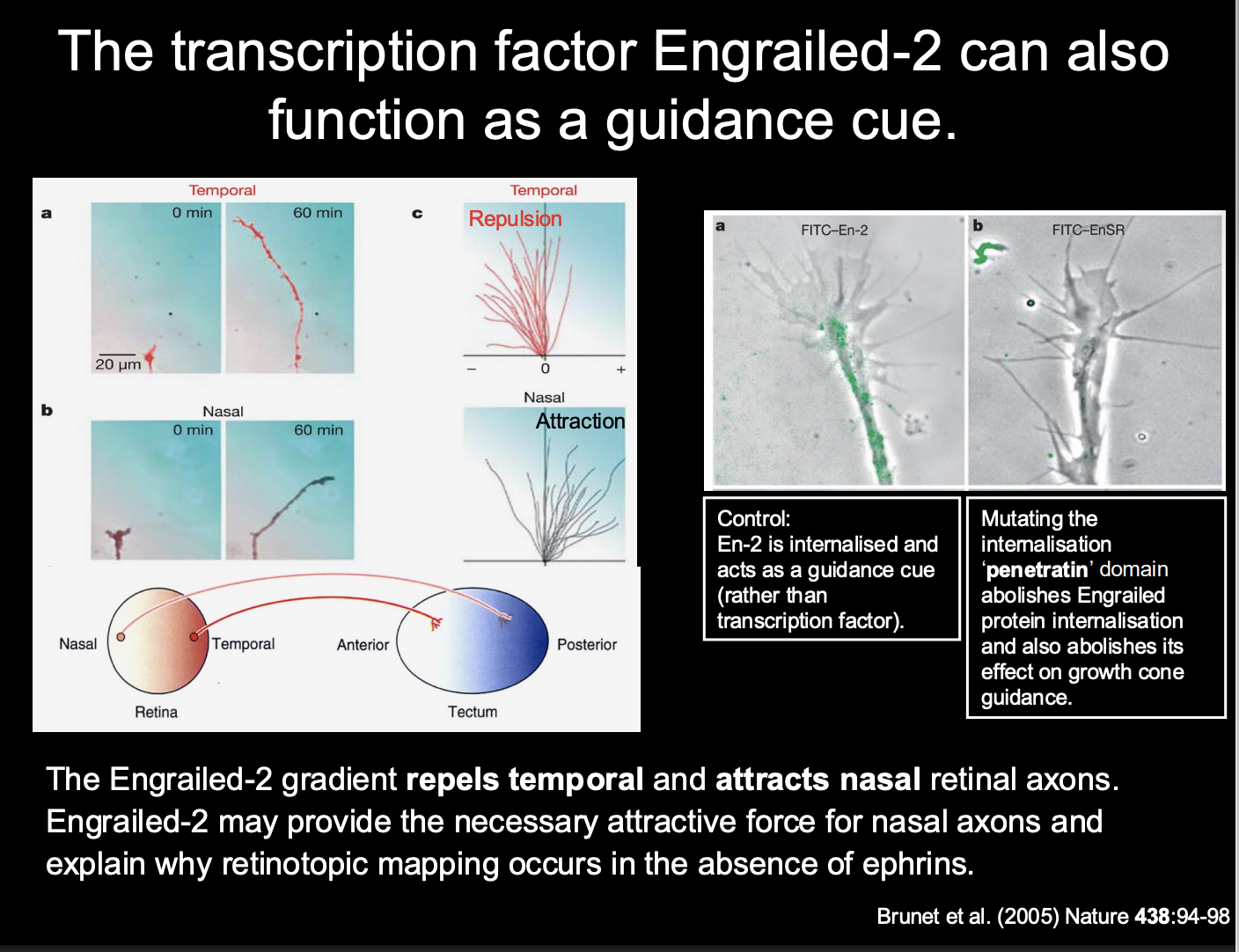
also acts as a guidance cue for retinal axons:
Taken up by growth cones of Xenopul reintal ganglion cells
initiates translation of new proteins and chemotropic response:
Temporal→ repulsion
Nasal→ Attraction
i.e→ we again get two forces from the same one gradient
How it was found out how Engrailed worked
Procedure:
Control→ En-2 internalised and acts as a guidance cue (rather tahn a TF
Mutating the internatision ‘penetratin’ domain→ abolish engralied protein internalisation→ abolishes effect on growth cone guidance
Conclusion
Engrailed is internalised→ so does act as a guidance cue and not as a TF in this instance
Therefore the presence of engrained 2 explains
how there is still mapping in the absence of ephrins
its attractive mechanisms may aid the precision needed
(which sn’t really provided by just repulsive mechanisms)
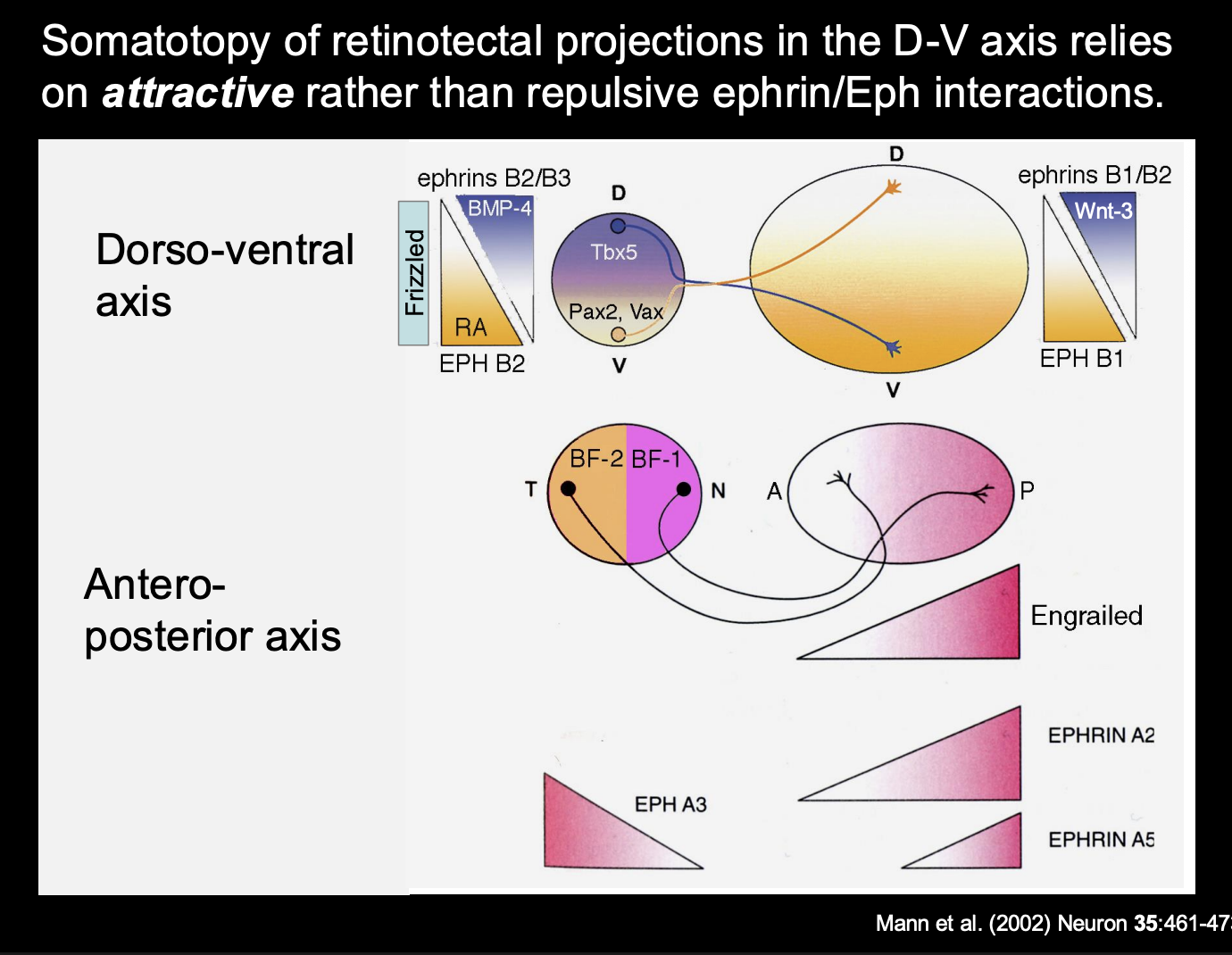
Patterning of retinaltectal projections in the D-V axis relies on
Attractive rather than repulsive ephrin/Eph interactions
Problem→ during development, the size and shape of the tectum changes
this means that even though there is retinotopy estblished at the start of development
this could be disrupted at the tectum itself grows
but→ it is not!
What does this suggested about the retino-topic connections made
have to be flexible
so they can be adjusted continually
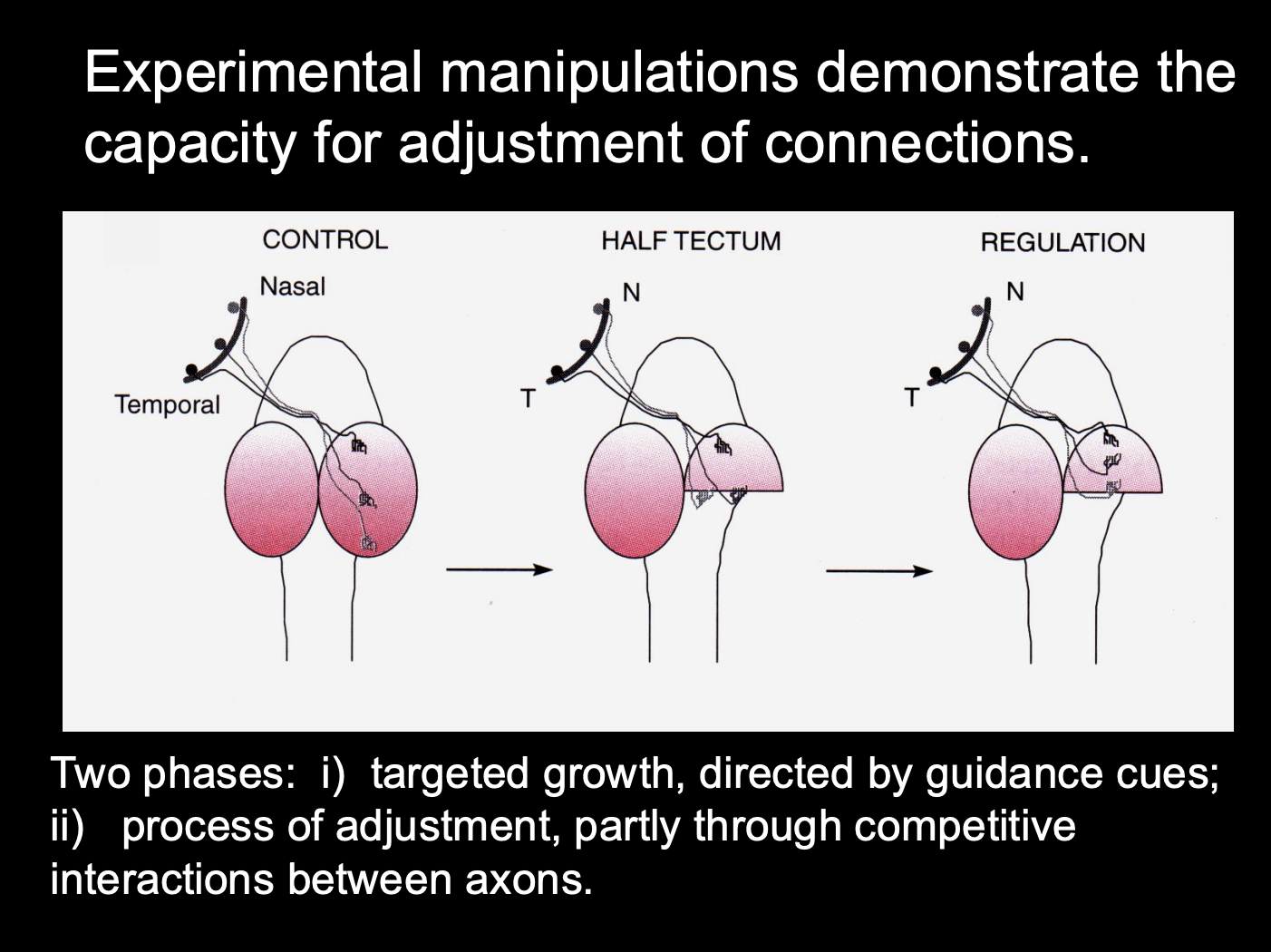
Experimental manipulations demonstrate the capacity for adjustments of connections
Procedure: see where axons grow in tectum manipulation
Control
Half tectum
Half tectum and then regulated with cues
Result:
Half tectum→ temporal axons want to not be in the posterior part (removes somatotopy)
regulated→ get somatotopy
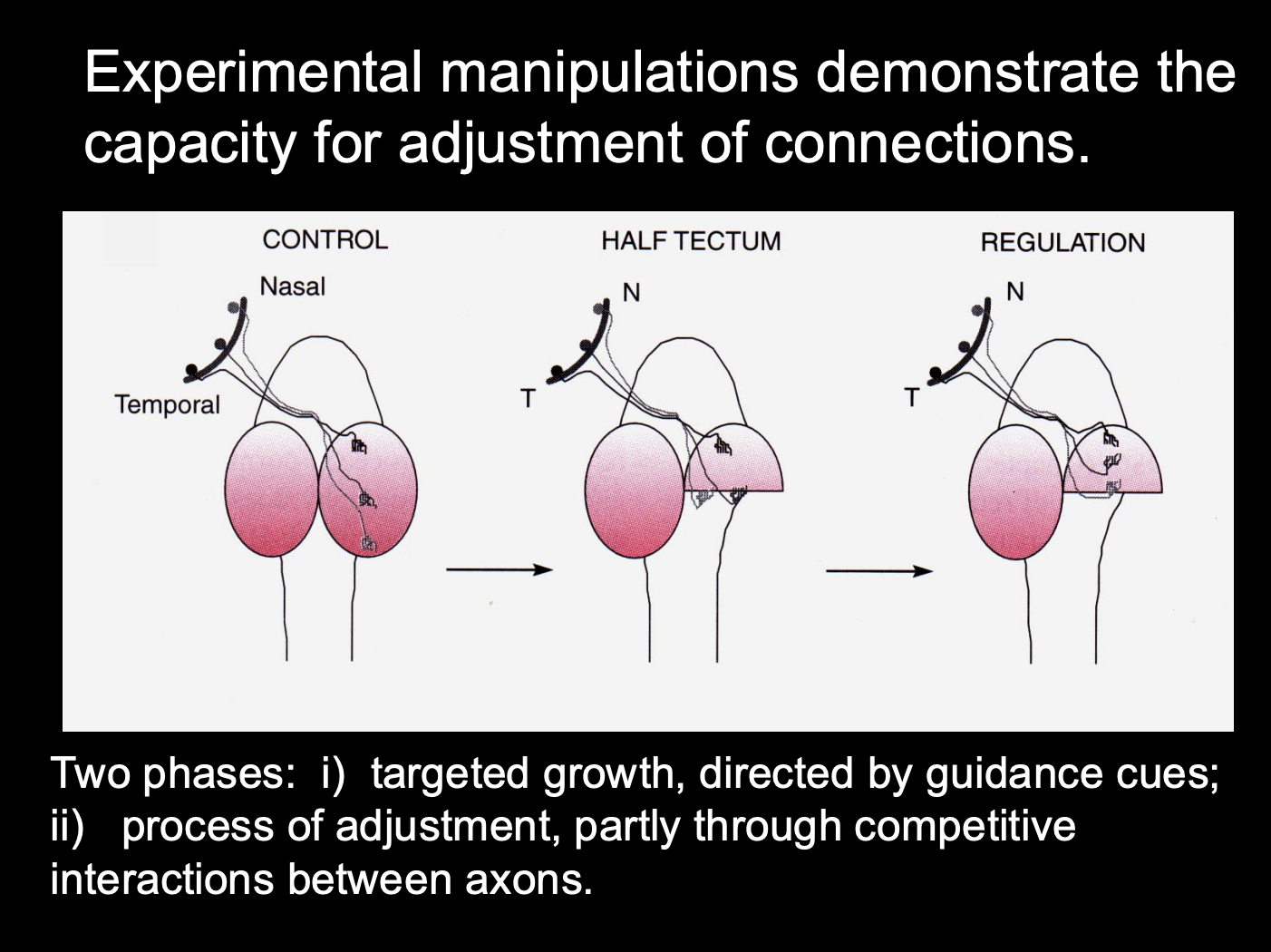
Experimental manipulations demonstrate the capacity for adjustments of connections→ retinal
Procedure: see where axons grow in retinal manipulation
Control
Nasal removed
Nasal removed and then regulated with cues
Results:
nasal removed→ none to the posterior
Regulated→ regains the somatotopy
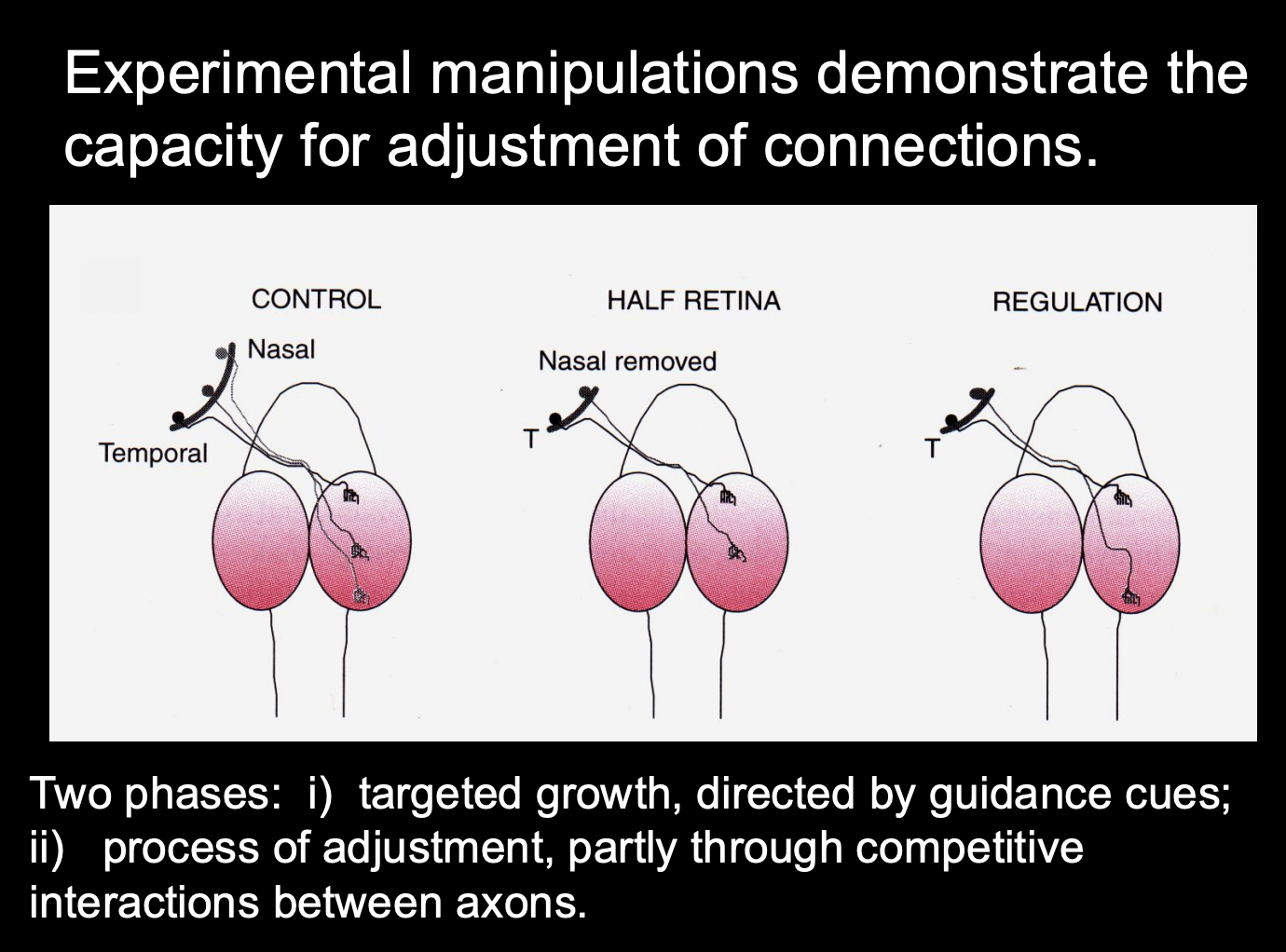
Two experiments show two pahses of this flexibilty
Targeted growth, directed by guidance cues
Process of adjustment, partly through compeitive interactions between axons
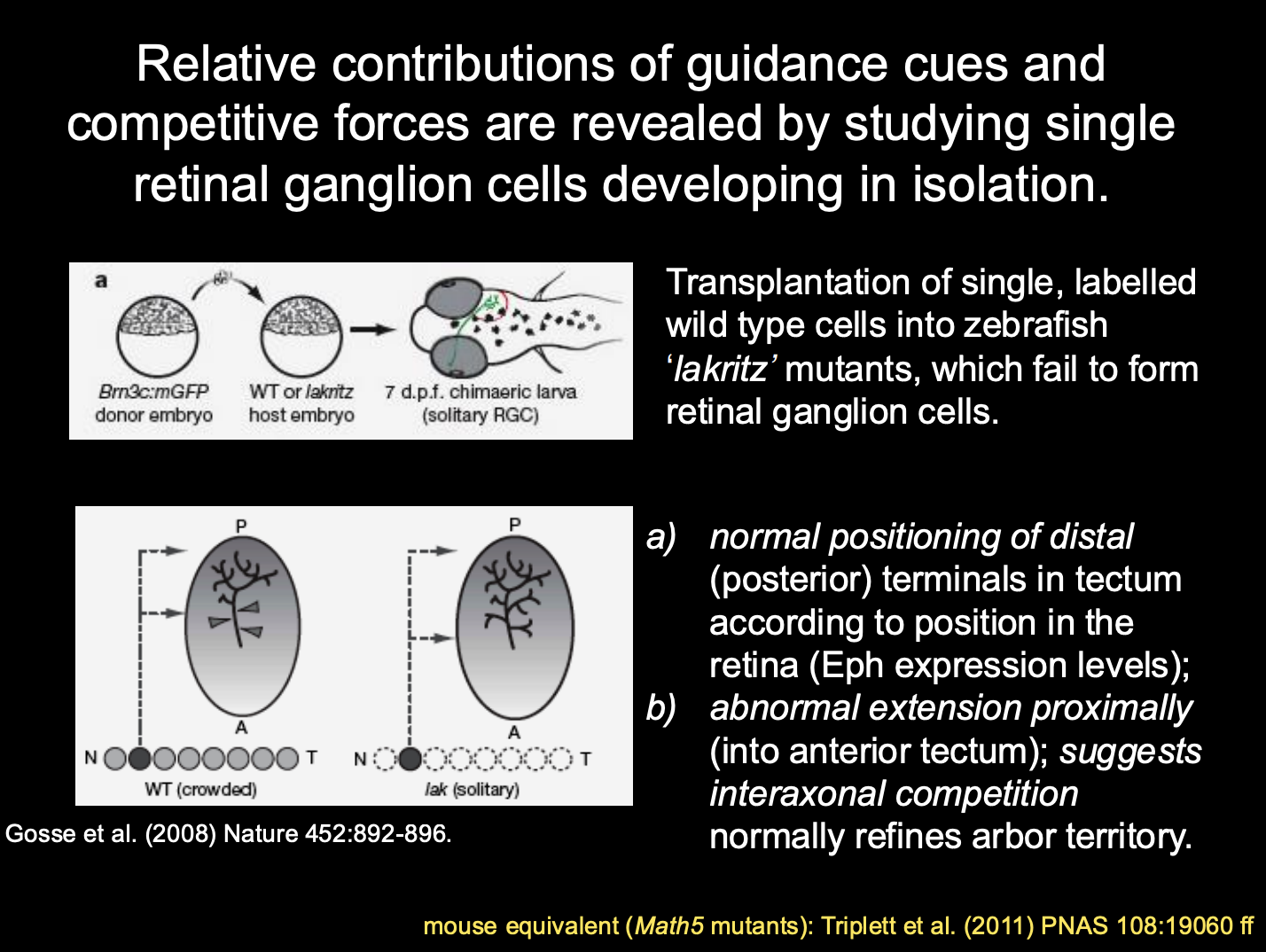
What are the relative contributions of these two forces (cues vs competition)→ how investigated
Procedure: studying single retinal ganglion cells develping in isolation
transplantation of single, labelled WT cells into zebrasih ‘lakritz’ mutants
these fail to form retinal ganglion cells
Results and conclusions
normal positional of distal (posterior) terminals in tectum according to position in the retina
→ Eph expression levels
Abnormal expression proximally (Into anterior tectum)
→ suggests: interaxonal competition normally refines arbor territory
Summary of this
retina and its target tissue are polarised
due to expression of patterns of
TFs and Guidance cues
What generates the retinotopic map in the early embryo
gradients of guidance cues and receptors
in retina and tectum/superior colliculus
(Eph/ephrin; Wnts;Engrailed)
What refines the coarse maps initially formed
neural activity and other compeitive mechanisms