4-5 GROUP
0.0(0)
Card Sorting
1/102
There's no tags or description
Looks like no tags are added yet.
Last updated 5:46 AM on 4/11/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
103 Terms
1
New cards
Ge
classified as metalloid in group 4
2
New cards
Carbon
serves as the basic building unit of organic compounds.
3
New cards
f
All carbons are organic
t or f
t or f
4
New cards
carbonates, oxalates, tartrates and acetates.
Carbon occurs in inorganic compound such as?
5
New cards
F
Is carbon dioxide organic?
\
t or f
\
t or f
6
New cards
Catenation-chain Formation
ability of carbon to form bonds with itself or chains; capable of forming four bonds.
7
New cards
2+ and 4+
\
ns2np2
\
ns2np2
The elements may exhibit _______oxidation states, in keeping their ________.
8
New cards
Isomerism
same molecular formula but different structure
9
New cards
Butane and isobutane
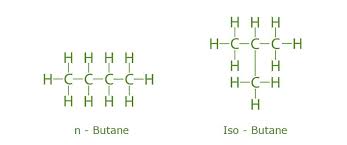
10
New cards
Hybridization
carbon can form hybrid orbitals; combination of orbitals to form hybrid orbitals
11
New cards
Sn and Pb
Metal in group 4
12
New cards
C, Si
Non-Metal in group 4
13
New cards
C
Fundamental constituent of all animal and vegetative tissue
14
New cards
Allotropes
different forms of an element in the same physical state; all solids for carbon
15
New cards
Diamond and graphite
Crystalline examples
16
New cards
Diamond
hardest mineral
17
New cards
graphite
the stable form of diamond
18
New cards
Bituminous , Anthracite
amorphous examples
19
New cards
Bituminous
soft coal
20
New cards
Anthracite
hard coal
21
New cards
Activated Charcoal
from ligneous material by carbonization in the absence of air followed by heat or chemical treatment to increase surface area.
22
New cards
Activated Charcoal
used in treatment of diarrhea
23
New cards
SILICON
2nd most abundant element on earth
24
New cards
Silica plates
made of SiO2 used in Thin Layer Chromatography
25
New cards
Silica
common name of SiO2
26
New cards
GERMANIUM
semi-conductors • used in microelectronic parts
27
New cards
Sn
**used in production of cans**
28
New cards
Stannous (Sn+2)
Stannic (Sn4+)
Stannic (Sn4+)
Forms two salts
29
New cards
LEAD
Most metallic element in the group IVA
30
New cards
Accumulative
long exposure to the poison that accumulated in the body and eventually manifested symptoms unless exposed to big amounts.
31
New cards
used as a **Protein precipitant**
32
New cards
Ethylenediaminetetraacetic acid, Plumbism / Saturnism
antidote and effects of Sn
33
New cards
CaEDTA
most common form used for lead poisoning.
34
New cards
memory loss
irritability
clumsiness o
projectile vomiting
irritability
clumsiness o
projectile vomiting
effects of Lead Encephalopathy
35
New cards
brain
Target Organ of Lead
36
New cards
Wrist drop / Foot Drop so called Pb Palsy
Neuro Effect of Lead
37
New cards
Metallic taste, black stools
GIT Effect of Lead
38
New cards
Microcytic, Hypochromic anemia
Blood description of people with Lead exposure
39
New cards
Fanconi-Like Syndrome
Instead of kidney reabsorbing the components back to the blood, it is released in the urine.
40
New cards
Paints
Canned Food – no longer used
Old Lead Pipes
Automobile Exhaust as tetraethyl lead - Now, it is Unleaded.
Canned Food – no longer used
Old Lead Pipes
Automobile Exhaust as tetraethyl lead - Now, it is Unleaded.
Functions of lead
41
New cards
Nitrogen and Phosphorus
non-metallic elements in group 5
42
New cards
Bi
metal in group 5
43
New cards
As and Sb
metalloids elements in group 5
44
New cards
NH3
All are flammable and poisonous except
t
t
45
New cards
t
All Group VA elements combines with hydrogen to form hydrides.
t or f
t or f
46
New cards
f
Ammonia is toxic in small doses
\
t or f
\
t or f
47
New cards
o Phosphine (PH3)
o Arsine (AsH3)
o Stibine (SbH3)
o Bismuthine (BiH3)
o Arsine (AsH3)
o Stibine (SbH3)
o Bismuthine (BiH3)
examples of hydrides in group 5
48
New cards
f
Bi has a tendency to accept electrons
\
t or f
\
t or f
49
New cards
Mephitic Air (“without life”), Azote
Common names of nitrogen
50
New cards
NITROGEN
non-reactive like noble gases
51
New cards
78-80%
amount of nitrogen present in the atmosphere
52
New cards
NITROGEN
used in refrigerant and cryogenic preservation.
53
New cards
NITROGEN
can freeze instantly for preservation of cells, tissues, and microorganisms.
54
New cards
Carbon dioxide
used in Laboratory euthanasia of animals
55
New cards
PHOSPHORUS
Essential constituent of protoplasm, nervous and bone tissue.
56
New cards
Light Carrier, St Elmo’s Fire
Common Names of phosphorus
57
New cards
Garlic Odor Breath
toxicity caused by phosphorus
58
New cards
Luminous Vomit
side effects of garlic odor breath
59
New cards
CuSO4 – Cupric sulfate
Treatment for garlic odor breath
60
New cards
White or Yellow
color of toxic phosphorus
61
New cards
o White/Yellow
o Black
o Red
o Violet
o Black
o Red
o Violet
allotropic forms of phosphorus
62
New cards
phossy jaw
Bony Necrosis especially mandible area called
63
New cards
rat poison
use of white phosphorus
64
New cards
Garlic Odor of Breath, Luminous vomitus, Severe GI irritation, Bloody Diarrhea, Liver Damage, General Protoplasmic poison
acute poisoning of white phosphorus
65
New cards
BLACK PHOSPHORUS
Resembles graphite in texture.
66
New cards
BLACK PHOSPHORUS
Produced from White P under High Pressure
67
New cards
BLACK PHOSPHORUS
Stable in air
68
New cards
BLACK PHOSPHORUS
Does not catch fire spontaneously.
69
New cards
RED PHOSPHORUS
Appears as red to violet powder.
70
New cards
RED PHOSPHORUS
Non Poisonous, Non Flammable type of phosphorus
71
New cards
t
White P is known to catch fire spontaneously
\
t or f
\
t or f
72
New cards
RED PHOSPHORUS
Less chemically active compared to White P
73
New cards
Safety Matches, Pyrotechnics
uses of red p
74
New cards
Dimercaprol also known as BAL (British Anti-Lewisite)
\
Antidote of Arsenic
Antidote of Arsenic
75
New cards
f
Arsenic in general is not toxic.
t or f
t or f
76
New cards
FeCl3 and Mg(OH)2
\
Ferric chloride and Magnesium hydroxide
\
Ferric chloride and Magnesium hydroxide
antidote for arsenic ingestion
77
New cards
Salvarsan/ Compound 606/ Arsphenamine
Arsenic is a component of what compound
78
New cards
ARSENIC
Lewisite metal contains
79
New cards
\
As+3 (**arsenic(III) cation)**
As+3 (**arsenic(III) cation)**
Arsenic is poisonous especially
80
New cards
helium
hydrogen
nitrogen
oxygen
carbon dioxide
hydrogen
nitrogen
oxygen
carbon dioxide
**Color coding of gas cylinders:**
Brown:
Red:
Black:
Green:
Grey:
\
Brown:
Red:
Black:
Green:
Grey:
\
81
New cards
BAL
antidote if arsenic was already absorbed in the GI tract
82
New cards
\
PARIS GREEN
SCHEELE’S GREEN
FOWLER’S SOLUTION
DONOVAN’S SOLUTION
PARIS GREEN
SCHEELE’S GREEN
FOWLER’S SOLUTION
DONOVAN’S SOLUTION
Compounds of Arsenic:
83
New cards
Cupric Acetoarsenite - Cu(C2H3O2) ● 3Cu (AsO2)2
PARIS GREEN also known as
84
New cards
PARIS GREEN
Cupric Acetoarsenite
Cu(C2H3O2)
3Cu (AsO2)2
Cupric Acetoarsenite
Cu(C2H3O2)
3Cu (AsO2)2
\
Use as a rodenticide, Insecticide, Pigment, and blue colorant for fireworks
Use as a rodenticide, Insecticide, Pigment, and blue colorant for fireworks
85
New cards
Arsenious oxide, white arsenic, Arsenic (III) Oxide, Arsenicum Album
Other name of arsenic trioxide
86
New cards
AsI3 and HgI2 Solution
DONOVAN’S SOLUTION:
87
New cards
1% Potassium Arsenite Solution; Anti Leukemic – K3AsO3
FOWLER’S SOLUTION:
88
New cards
Cupric Hydrogen Arsenite – AsCuHO3
SCHEELE’S GREEN:
89
New cards
ARSENIC TRIOXIDE
treatment for leukemia
90
New cards
General Protoplasmic Poison
effect of Arsenic poisoning
91
New cards
Digestive Problems: Vomiting, Abdominal Pain, Diarrhea accompanied by bleeding, Hair loss
cause of acute poisoning in arsenic
92
New cards
Mees Lines
Characteristic White line in Nails
93
New cards
Arsenicosis and Cancer
causes of chronic poisoning in arsenic
94
New cards
Antimony Glance (Sb2S3) - Antimony sulfide
Most important source of Antimony
95
New cards
Antimony
Red Orange in Color
96
New cards
Astringent
Emetic
Expectorant
Anthelmintic (for Schistosomiasis) - Kills and Expel intestinal worms.
Emetic
Expectorant
Anthelmintic (for Schistosomiasis) - Kills and Expel intestinal worms.
Pharmacologic actions of antimony
97
New cards
Vermifuge
\
The anthelmintic drug that expel worms
The anthelmintic drug that expel worms
98
New cards
Antimony potassium tartrate - K2Sb2(C4H2O6)2
important compound of Sb
99
New cards
Brown mixture
Tartar emetic common name
100
New cards
Schistosomiasis
What disease is Antimony potassium tartrate for