Required Practical 1: Titrations
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Method for measuring mass accurately
Measure the mass of weighing bottle with the required quantity solid in it on a 2d.p or 3d.p balance
Empty the solid into a flask etc. (wtvr ur using in the experiment)
Reweigh the now empty weighing bottle
Subtract the mass of the first reading from the mass of the empty weighing bottle to give the exact mass of solid actually added
Method for diluting a solution up to 250cm³
Pipette 25.0cm³ of original solution into a 250cm³ volumetric flask
Make up to the 250cm³ mark with distilled water, using a dropping pipette for the last few drops
Invert flask several times to ensure uniform solution
Advantage of using a volumetric pipette over a measuring cyclinder
Volumetric pipette is more accurate as it has a smaller uncertainty
Give one reason why you shouldn’t heat or put hot solutions in a volumetric flask
The heat would cause the flask to expand, so the volume would then be incorrect
Method on how to make a 250cm³ solution
Weigh the sample bottle containing the solid on a balance
Transfer the solid to beaker and reweigh the now empty sample bottle
Record the difference in mass
Add distilled/deionised water to the beaker and stir with a glass rod until all solid has been dissolved
Transfer this solution to a volumetric flask using a funnel
Rinse the beaker and funnel with distilled/deionised water
add the washings from the beaker and glass rod to the volumetric flask
make up to 250cm³ with distilled/deionised water and then invert the flask several times to ensure uniform concentration throughout the solution
How to make up a volumetric solution and carry out a simple acid-base titration
Rinse equipment
burette with acid, pipette with alkali and conical flask with distilled water
Pipette 25 cm³ of alkali into conical flask
Touch surface of alkali with pipette to ensure correct amount is added
Add acid solution from burette
make sure the jet space in the burette is filled with acid
Add a few drops of indicator and refer to colour change at end point
Place a white tile underneath the flask to help observe the colour change
Add acid to alkali whilst swirling the mixture and
add acid drop wise near the end point
note burette reading before and after addition of acid
repeats titration until at least 2 concordant results are obtained
Why is conical flask used in preference to a beaker?
Because its easier to swirl the mixture in a conical flask without spilling the contents
What would happen if the jet space was not filled properly?
would lead to errors if it fills during the titration, leading to a larger than expected titre reading
Why should u remove the funnel from the burette?
because small drops of liquid may fall from the funnel into the burette during titration
leading to a false burette reading
would give a lower titre volume
what are indicators and why should you not add too much of them?
Indicators are generally weak acids so only add a few drops of them
If too much is added they will affect the titration result
Why may distilled water be added to the conical flask during a titration?
To wash the sides of the flask so that all the acid on the side is washed into the reaction mixture in order to react with the alkali
adding water does not affect titration reading as the water doesn’t react with reagents, or change the number of moles of acid added
What can we say lf 2 or 3 values are within 0.10cm³ and therefore concordant
We can say
results are accurate and repeatable
titration technique is good and consistent
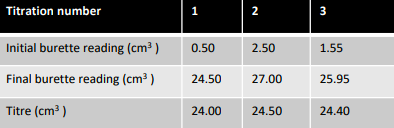
How should titration results be recorded?
Results should be clearly recorded in a table
Result should be recorded in full
i.e. both initial and final readings
Record titre volumes to 2d.p 0.05 cm³