chem
1/141
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
142 Terms
atom
smallest unit of matter that possesses the properties of an element – building blocks of matter
element
substance containing only one type of atom – its properties are determined by the number of protons in the atom
molecule
unit made up of two or more atoms
diatomic
molecule made up of two of the same type of atom
compound
substance made up of two or more elements
determines the element
number of protons in an atom
isotopes
atoms with the same number of protons but different number of neutrons
electrons are
negative
protons are
positive
neutrons are
have no charge
determines the mass of an element
neutrons and protons
1 mol (Avogrado’s number)
6.022×10²3
molar mass
average isotopic atomic weight in grams
mass-mol-atom conversion

atomic oxygen
O
molecular oxygen
O2
measured mass
m=V(p)
solution
a homogeneous mixture of substance composed of at least one
solute and one solven
homogenous mixture
uniform mixture of only one phase (one substance)
solute
substance that is dissolved in a solvent
solvent (aq)
refers to water – usually present in much larger quantities than the solutes (Note: solvents need not be liquid
aqueous
refers to types of solutions that have water as the solvent. Water is the most common solvent, with even blood plasma as an “aqueous solution”.
types of aqueous solutions
Electrolytic Solutions and Non-electrolytic solutions
Electrolytic Solutions
solutions that can conduct electricity – usually due to a dissociation reaction during dissolution resulting in individual charged cations and anions
non-electrolytic solutions
solutions that do not conduct electricity – in such solutions, the dissolved molecules are electrically neutral and remain intact
example of electrolytic solution
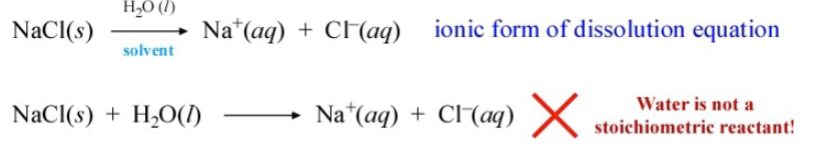
stoichiometric reactant
is a reactant that is consumed in a reaction
representation of a dissociation reaction
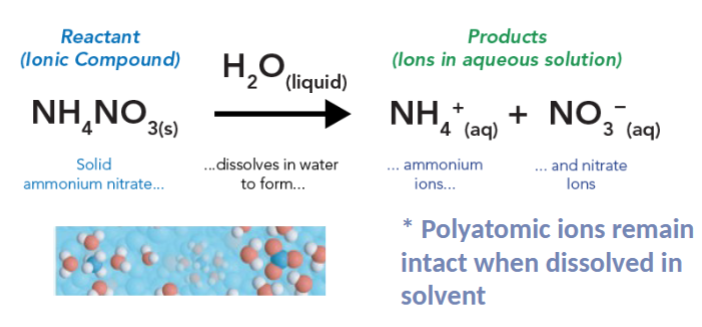
example of non-electrolytic solution
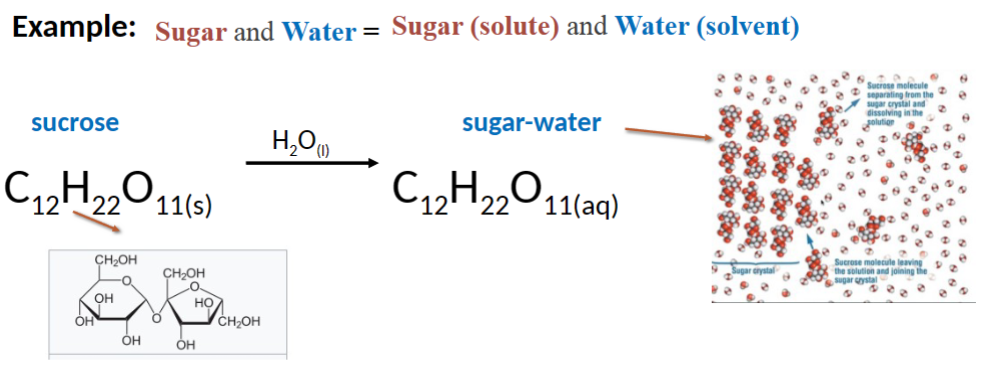
concentration
amount of a solute dissolved in a given volume of solvent (expressed as mass/volume or moles/volume).
Concentrated vs. dilute solutions (aq)
concentrated solutions have a large amount of solute dissolved in a volume of water, while dilute solutions have a small amount of solute. a vendor)
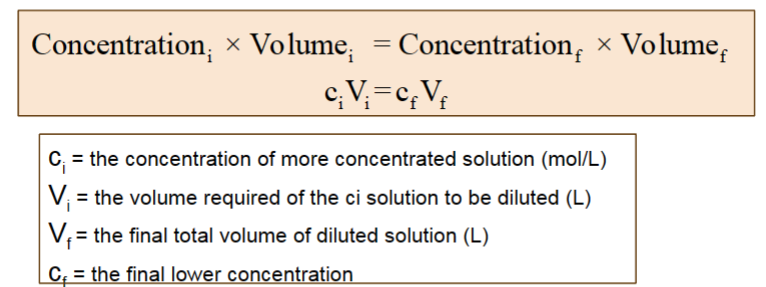
dilution (aq)
process of making a solution of lower concentration from a solution of higher concentration, by adding more solvent (water)
standard solution
prepared solutions of known concentration
how are standard solutions prepared
prepared by diluting more concentrated solutions, called stock solutions.
stock solutions
will be of sufficient concentration to be able to make several
volumes of more dilute standard solutions and will also be of known concentration.

moles as concentrations
concentration is moles/L
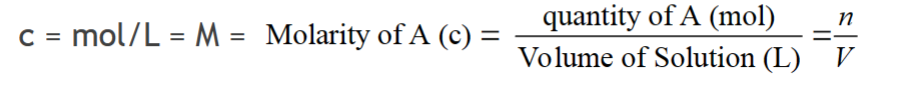
dilution
stoichiometry
the relationship between the relative quantities of substances taking part in a reaction or forming a compound, typically a ratio of whole integers (i.e., the mole ratios from a balanced equation
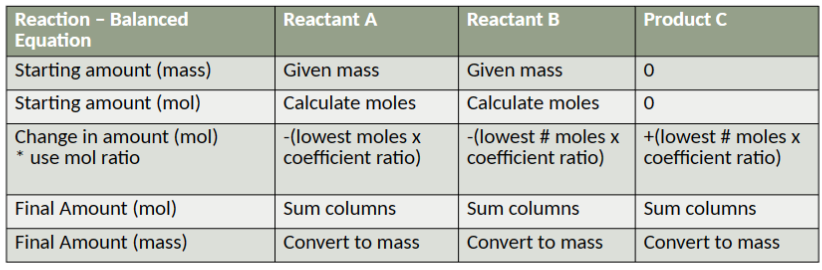
stoichiometric ratios help determine
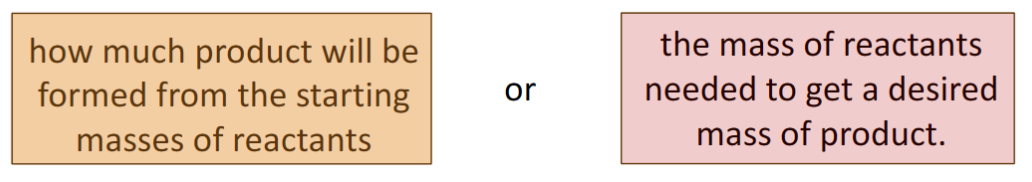
convert the mass of one substance to the mass of another
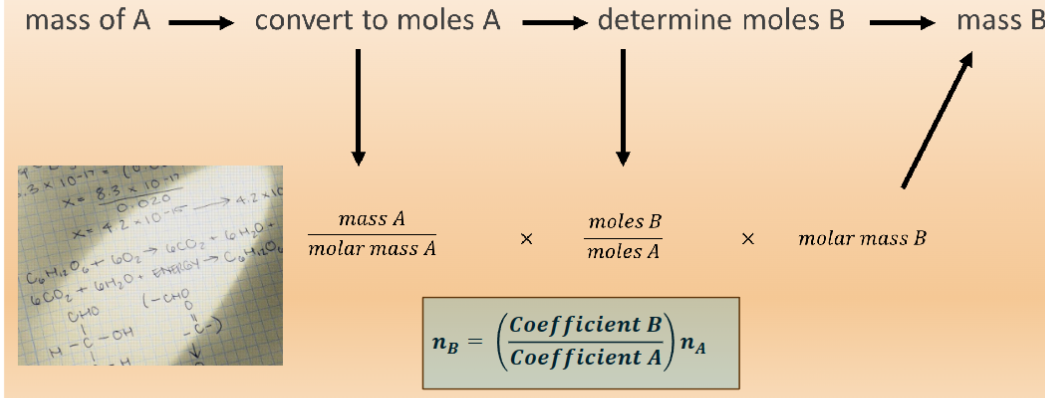
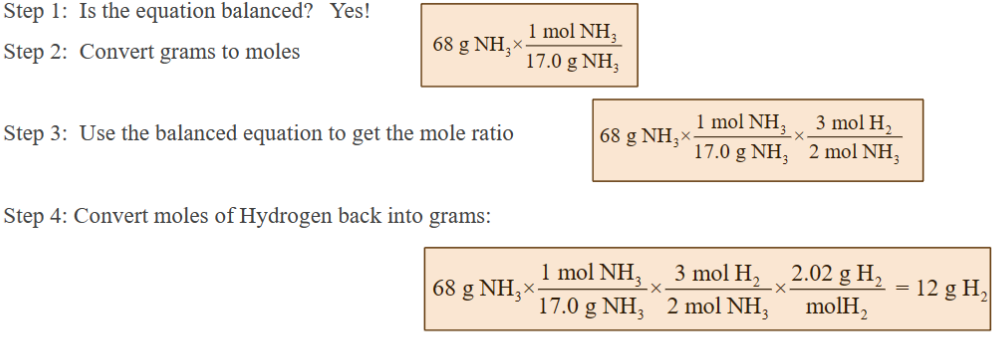
example: how many grams of hydrogen are need to produce 68 g of ammonia?
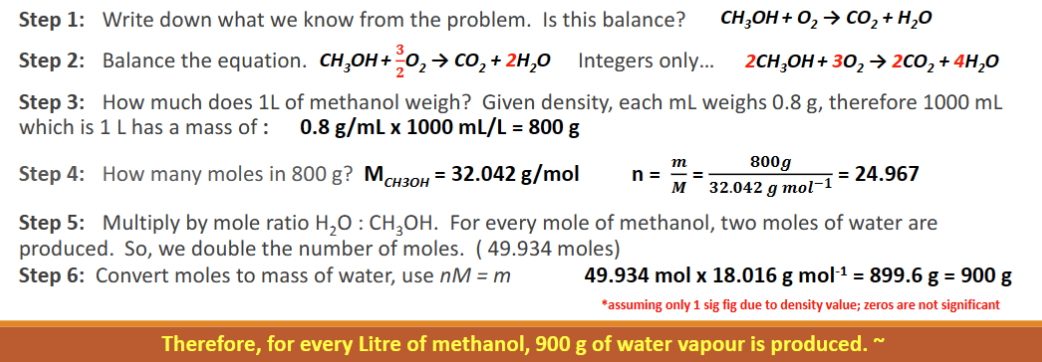
Some racing cars use methanol, CH3OH, as their fuel. What mass of water results from every Liter used (density = 0.8 g/mL)
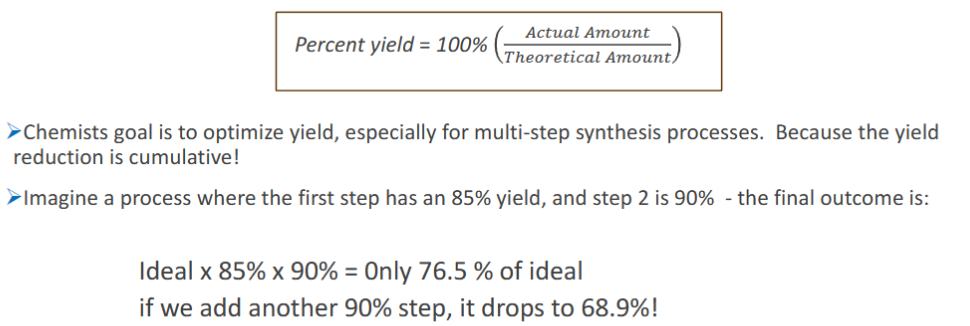
percent yields
main reasons for incomplete reactions

theoretical yield
the amount of product we would get if all reactants were converted entirely to products completely with nothing leftover (maximum amount in a perfect world)
actual amount
the quantity of product actually obtained from a chemical
reaction in the lab
Imagine a synthesis with two steps needed to make the desired product. We’re told the first step has an 85% yield, and the 2nd has a 90% yield. Calculate the final yield:
After 1st Step: Ideal x 85% = 0.85 of Ideal yield
After 2nd Step: 0.85 Ideal x 90% = 0.69 of Ideal yield
* You can see how fast the yield is dropping with each successive step
yield reduction is
cumulative
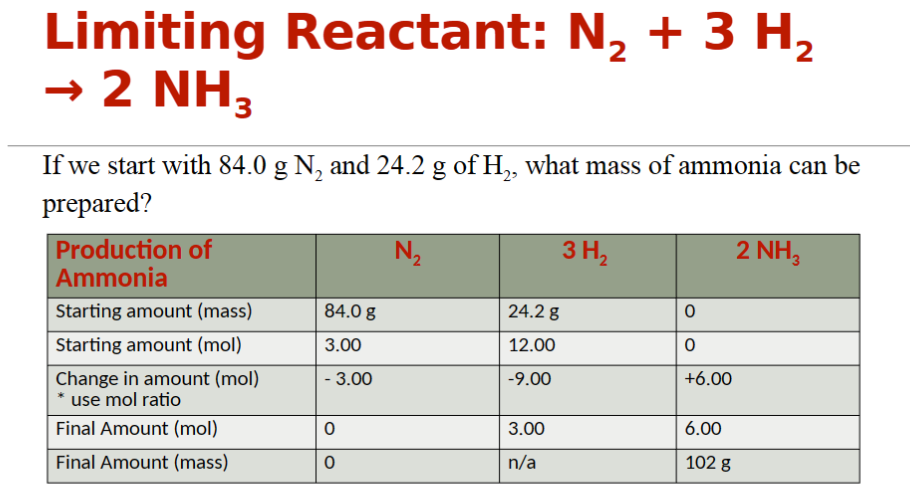
limiting reactant
The “leftover” reactants are said to be excess. The limiting reagent directly affects the potential yield for a reaction.
how to find the limiting reactant
use the stoichiometric ratios and compare the number of moles of each starting material available against the number of moles required to make the desired product


limiting reactant example
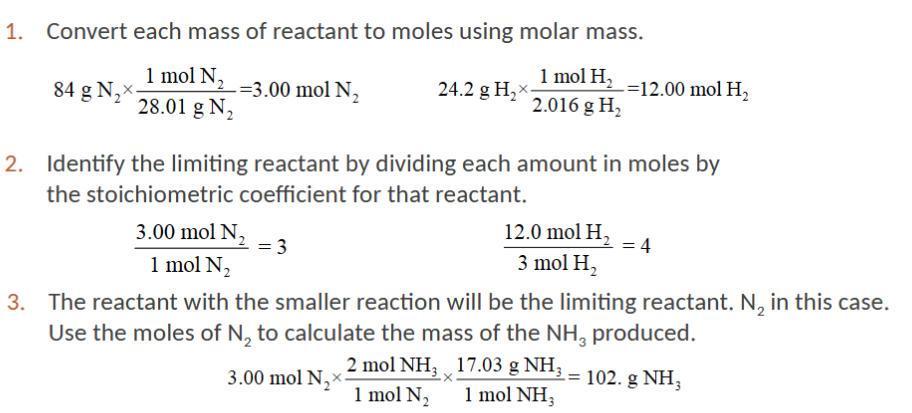
limiting reactant table example
law of conservation of mass
atoms cant be subdivided, created nor destroyed
law of constant composition
atoms are combined, separated ir rearranged i constant ratios in chemical reactions
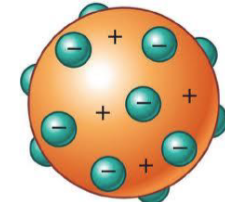
j.j thompson
plum pudding atom - identified negatively and positively charged pieces of an atom → subatomic particles (electron, proton)
first evidence of isotopes (same element, different
number of neutrons)he theorized that electrons were embedded in the atom like chocolate chips in a cookie
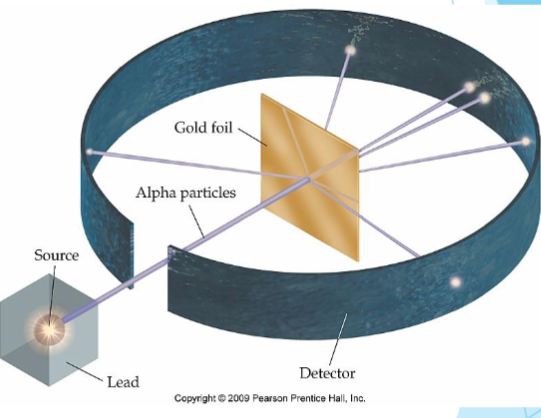
rutherford gold foil experiment
He arranged a Sn detector around a piece of gold foil. He directed a beam of alpha particles (protons) at the gold fold.
particles were deflected at wide angles, and some were reflected back towards the detector.
atomic model
the nucleus is surrounded by the electrons
with mostly empty space in between
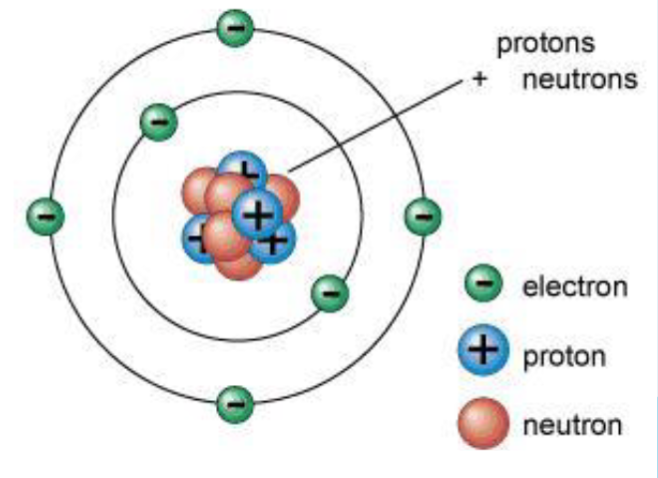
modern atomic orbital theory
diffuse electron cloud around nucleus (if we consider the relative size of the nucleus to the electron cloud, we see that if the electron cloud were the size of a football stadium, the nucleus would be the size of a pea.
- specific shapes and probability of locations for different levels.
- uses Heisenberg Uncertainty Principle to define states of electrons.
characteristics of atoms
have
mass and volume
positive nuclei, charge Z
electrons (determine properties of an element)
unique physical and chemical properties
atoms attract one another and can combine
atomic values example

light - EM radiation in 400-700 nm range
is a form of electromagnetic radiation
behaves as a wave
composed of photons (particles)
interacts with atoms in a predictable and diagnostic way
Waves: repeating oscillations
wavelength
the distance between two successive crests (units: meters or nanometers)
frequency
the number of waves passing a certain point over a unit of time (units: s−1 = Hz)
amplitude
height of the wave measured from the axis of propagation, a measure of intensity
amplitude and intensity
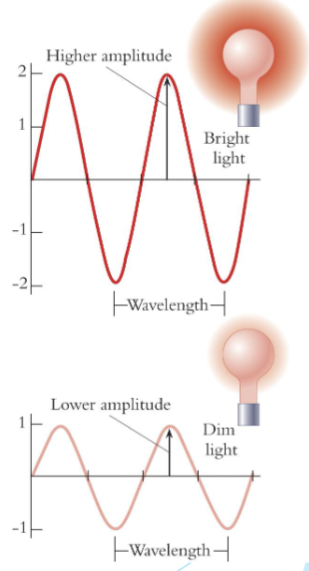
light is a
wave and has energy measured in J
photoelecric effect
Below a certain frequency, no electrons were
observed, no matter what the intensity.The energy of the ejected electrons increased
linearly with the frequency of light.The number of emitted electrons increased with
light intensity.All metals show the same pattern, but each
metal has a different threshold frequency.
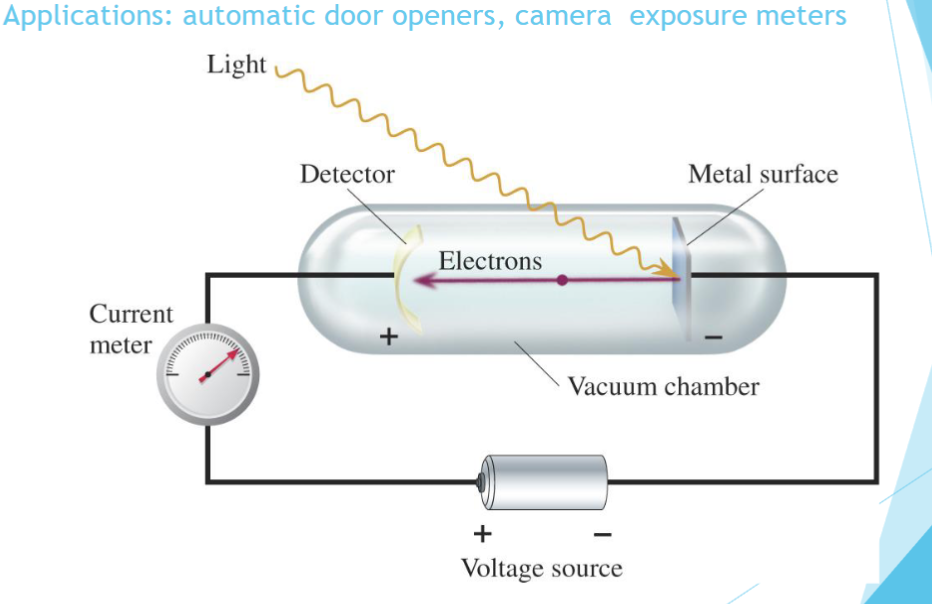
Electrons are only ejected if
the frequency of light is high enough.
the greater the intensity of light
the more photons, but if no individual photon has enough
energy (a high enough frequency) to remove an electron, then none are ejected
planck equation
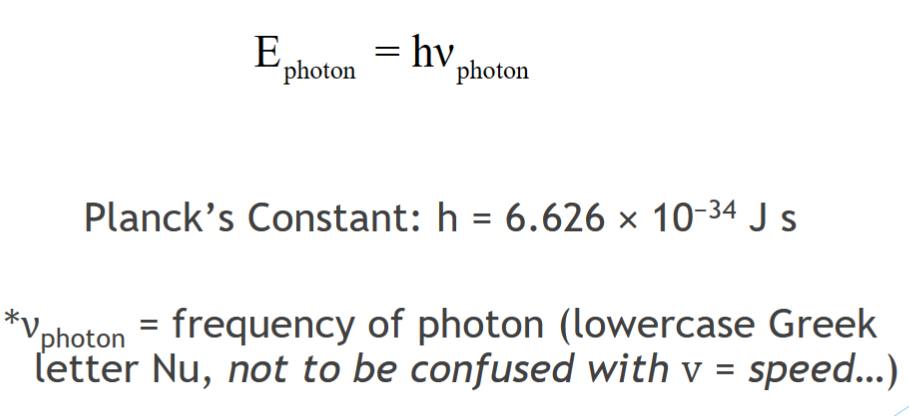
When a metal surface absorbs a photon
the energy is transferred to an electron.
binding and kinetic energy
Electron kinetic energy = Photon energy – Binding energy
Some of the energy must be used to cover the forces that bind the electron to the metal.
The remainder shows up as the kinetic energy of the ejected electron.
typical results when light interacts with electrons
Photoionization: energy high enough to eject an
electronNo ionization: atoms can gain energy, but do not
ionize (light has lower energy than threshold)
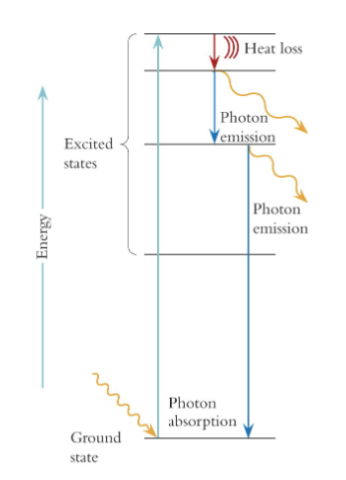
excited state of an electron
are unstable
will re-emit the excitation energy as light, heat, or
motion (kinetic energy)re-emitting the excitation energy returns the atom to ground state
ground state is an atom’s most stable state
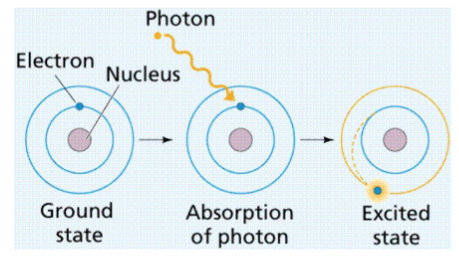
absorption and emission spectra
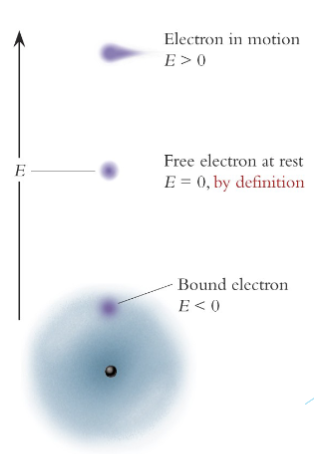
Need a way to represent the energy of the bound electron to the energy of the excited electron.
Convention is to compare it to a hypothetical “free” electron at rest, whose energy is deemed to be 0.
Bound electron will have negative energy compared to this.
Excited electron will have positive
energy compared to “free” electron.
ground state
lowest energy state of an atom
excited state
when an atom absorbs a photon
energy level diagram
depicts the changed in energy of a atom
When an atom emits a photon (or radiates heat)
it returns to the ground state
absorption
Usually, a source of white light passes through a
sample, and the atoms absorb specific
frequencies of light.
An absorption spectrum measures
the frequencies of photons that an atom absorbs
emission
When excited atoms emit photons, the frequencies of the photon are specific.
An emission spectrum plots the intensity of light as a function of frequency.
niels bohr
used planet model - electrons orbiting around the
nucleus.electrons can only occupy certain energy levels;
quantized levels.
energy state potential energy model
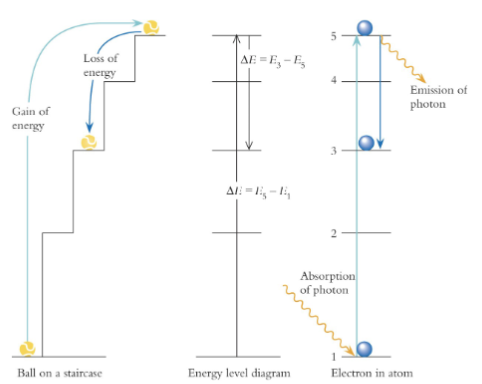
absorption/emission
If the atom absorbs a photon = energy of atom increases.
ΔE is positive : final energy is higher than initial energy
If the atom emits a photon = energy of atom
decreases.
ΔE is negative : final energy is lower than initial energy
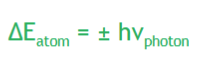
electron “cloud”
Not an amorphous cloud, but actually is described by the probability of where an electron could be at any given point in time. These areas of probability are discrete and “quantized” - That is, an electron cannot exist in between these regions.
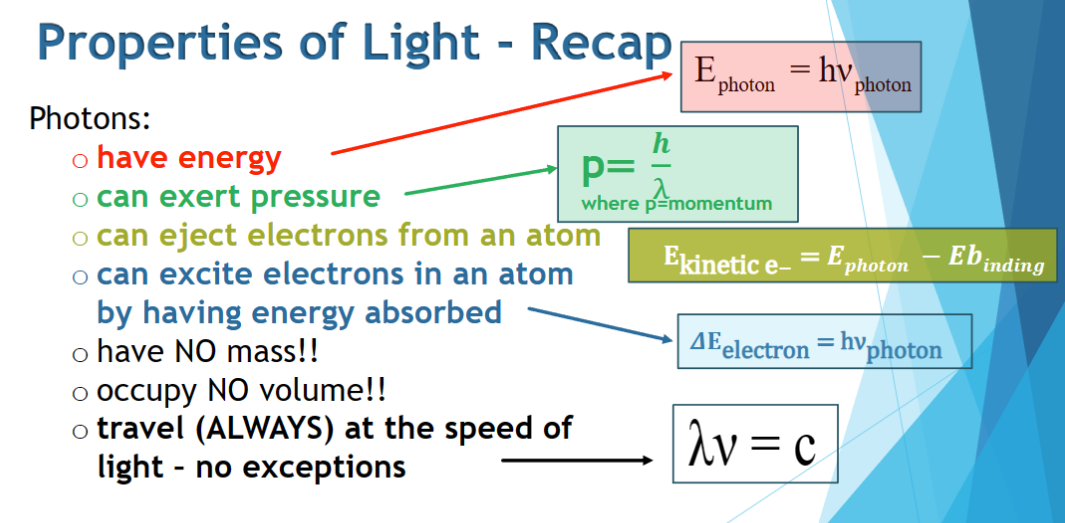
properties of light
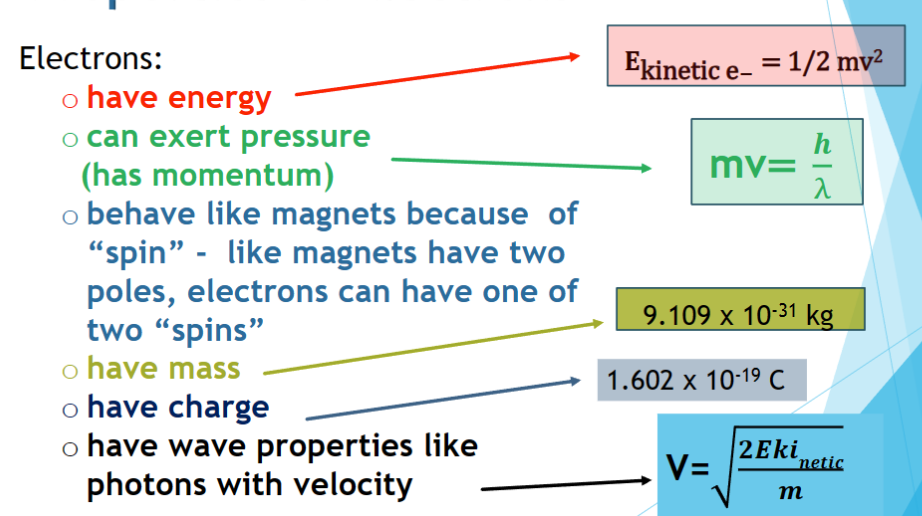
properties of electrons
orbital names
The names of orbitals from early
spectroscopic measurements.
s = “sharp”
p = “principle”
d = “diffuse”
f = “fundamental”
orbital
A region of probability where an electron will exist at a specific
energy level. Its shape can be represented with an Electron Density Plot
shell
A group of atomic orbitals with the same value of Principal
Quantum Number, n
subshell =
orbital – denoted by letters s, p, d, f, etc. Each subshell has a
predictable 3D shape, a quantized energy level, and can accommodate a specific number of electrons
valence electrons
those electrons that occupy the highest shell of an atom and are the electrons that form chemical bonds with other atoms
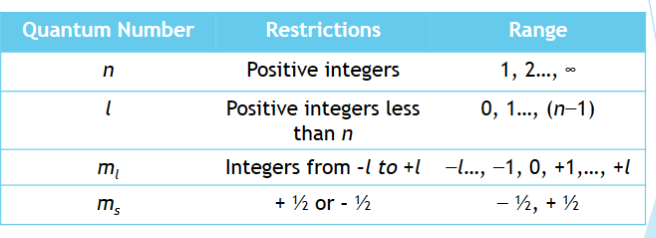
quantum numbers
a unique group of 4 numbers assigned to describe the state of an individual electron in an atom. Can be considered the “address” of the electron on the atom
principal quantum number (n)
a number specifying the theoretical energy level of and electron in an atom – refers to the “shell” the electron inhabits
(n= 1, 2, 3, 4 etc)
azimuthal Quantum Number (l)
the number referring to the orbital in which an electron resides (s=0, p=1, d=2, f=3, etc.)
Magnetic Quantum Number (ml))
is the quantum number that denotes the orientation of an orbital in space on the x-y-z 3D axis (between +l and –l)
Spin Quantum Number (ms)
the quantum number that denotes the spin of an electron. The values can only be + ½ or - ½ .
restrictions on quantum numbers for atoms