IB Biology: Cell Respiration
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
Cellular respiration
the controlled release of energy from breaking down organic compounds to produce atp
ATP benefits:
releases small amount of energy- enough to drive metabolic reaction while keeping energy wastage low
can be recycled- reversible breakdown of cell
Hydrolysis is quick and easy- allows cells to respond to a sudden increase in energy demand
soluble- easy movement IN the cell
How is mitochondria adapted for ATP
synthesis:
cristae - high SA: VOL - higher rate of diffusion
double membrane - intermembrane space - electrocgemical H+ gradient
Uses of atp:
Role of ATP Synthase
An enzyme that diffuses H+ ions across the cristae into the intermembrane space down the conc. gradient.
Catalysing the phosphorylation of ADP or ATP
Equation for reduction of NAD+
NAD+ + 2e- + 2H+ → NADH + H+
Reduction of FAD equation:
FAD + 2e- +2H+ → FADH2
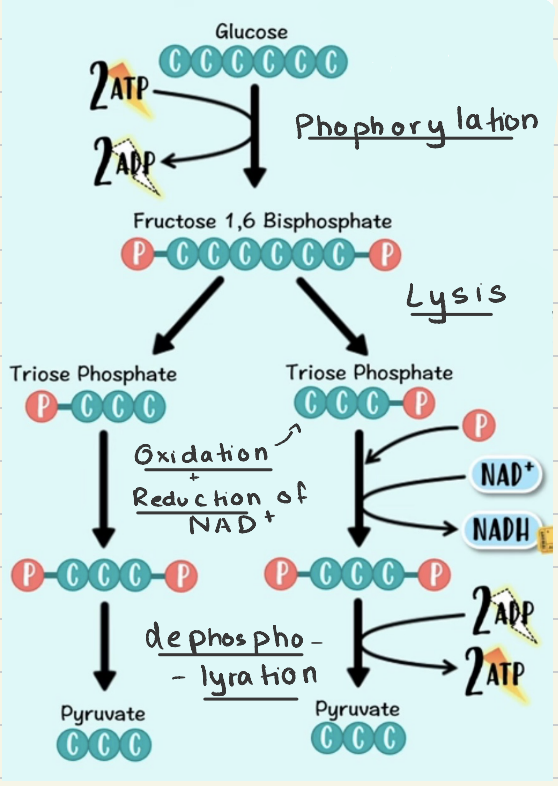
First step of respiration- cytoplasm
Phospholyration: glucose (6C) + 2ATP → Fructose- 1,6 - Biophosphate
Lysis: 6C → 3C
Oxidation: 2 Triosephosphate → 2 Glycertate-3-phosphate
Reduction: 4H + 2NAD+ → 2NADH + 2H+
ATP Formation: phosphates are transferred from intermediate substrates
4Pi + 4ADP → 4ATP
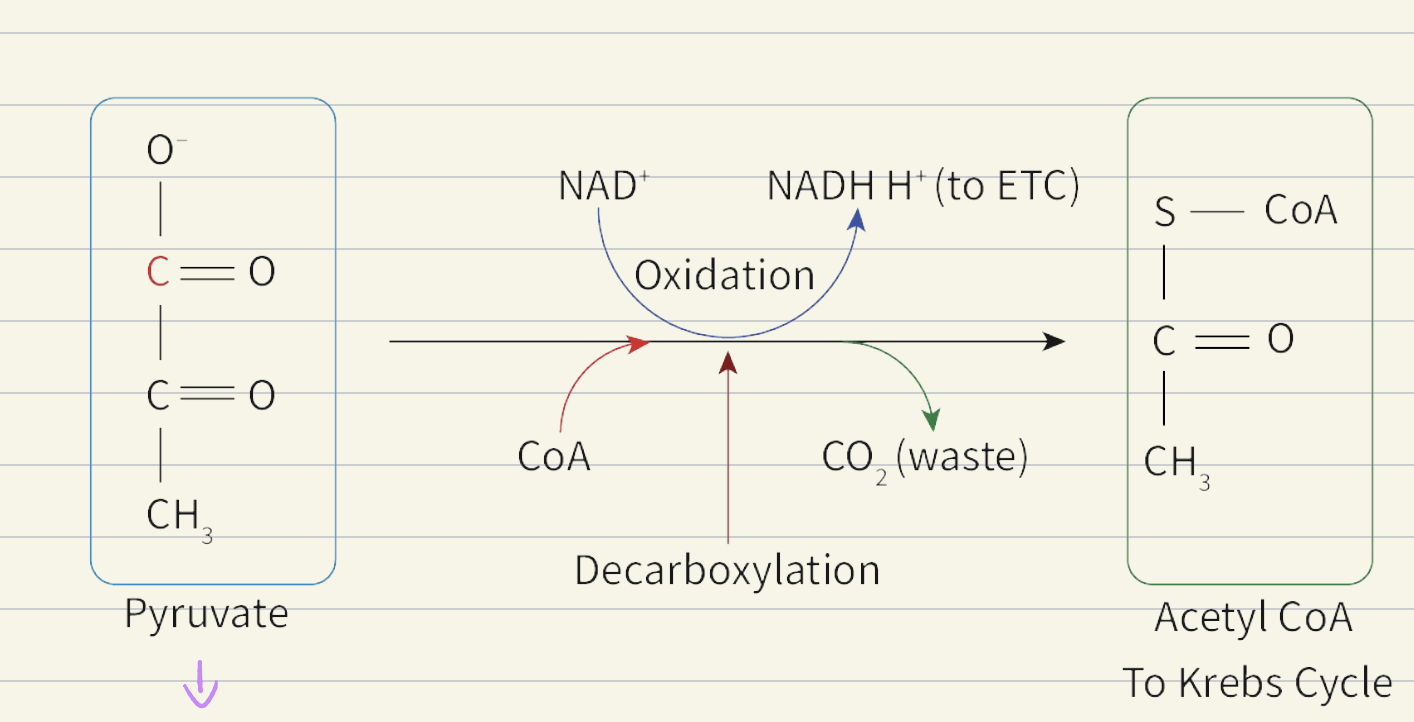
Link reaction:
links lycolysis to krebs cycle
Pyruvate (3C) decarboxilised to form Acetyl (2C)
Acetyl oxidised (loses its H) and NAD+ is reduced H + NAD+ → NADH + H+
Acetyl combine with coenzyme A to form Acetyl CoA (2C)
Since 2 pyruvate, reaction x2
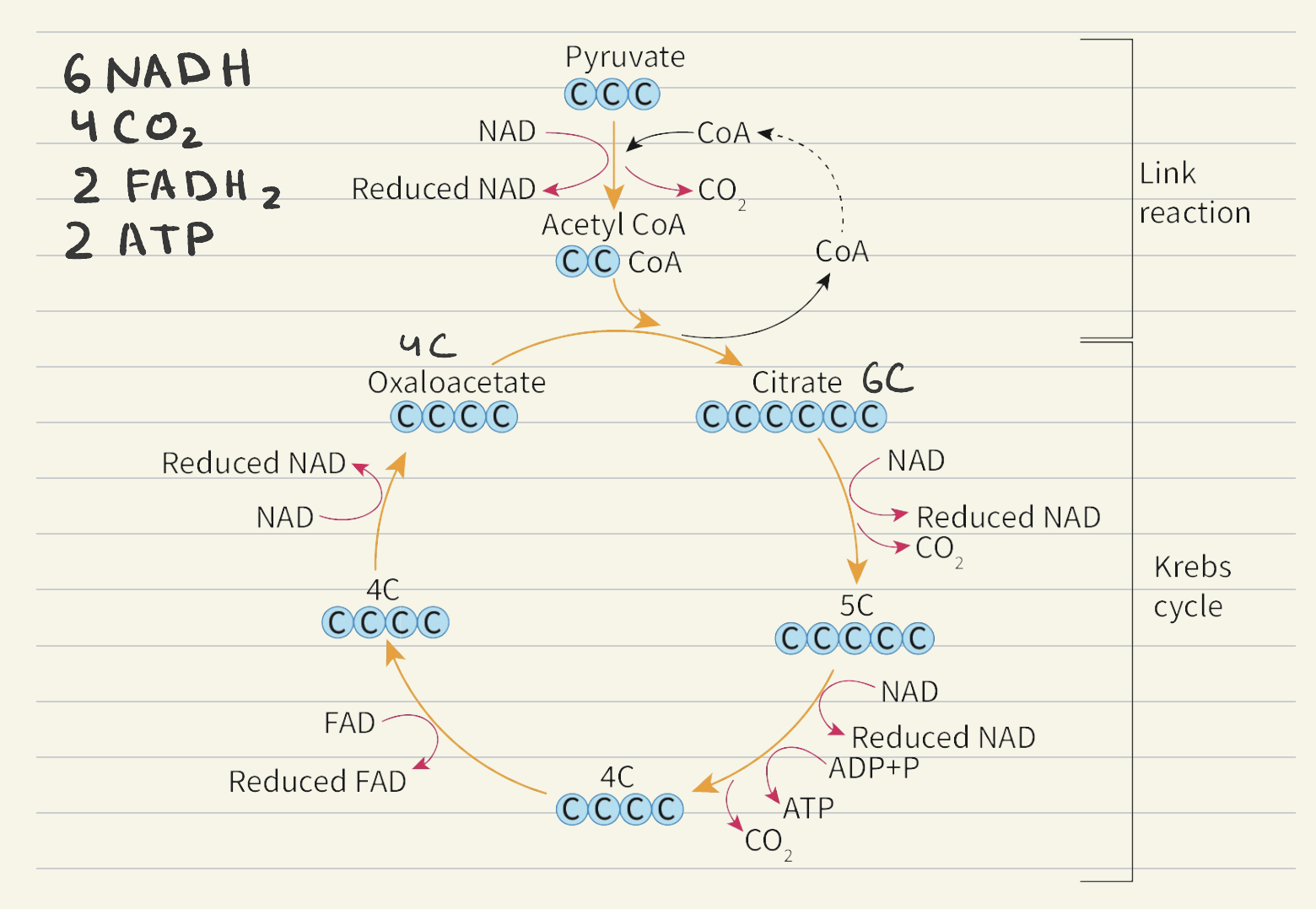
Krebs cycle:
Acetyl CoA (2C) combines with Oxaloacetate (4C) to form Citrate (6C) - CoAis recycled back to link reaction
Citrate(6C) is oxidised and NAD+ is reduced
Citrate(6C) is decarboxylised
5C is oxidised and NAD+ is reduced
5C is decarboxylised
ATP ← ADP + P
4C is is oxidised and NAD+ is reduced
FAD is reduced to FADH2
Cycle repteats with oxaloacetate (4C)
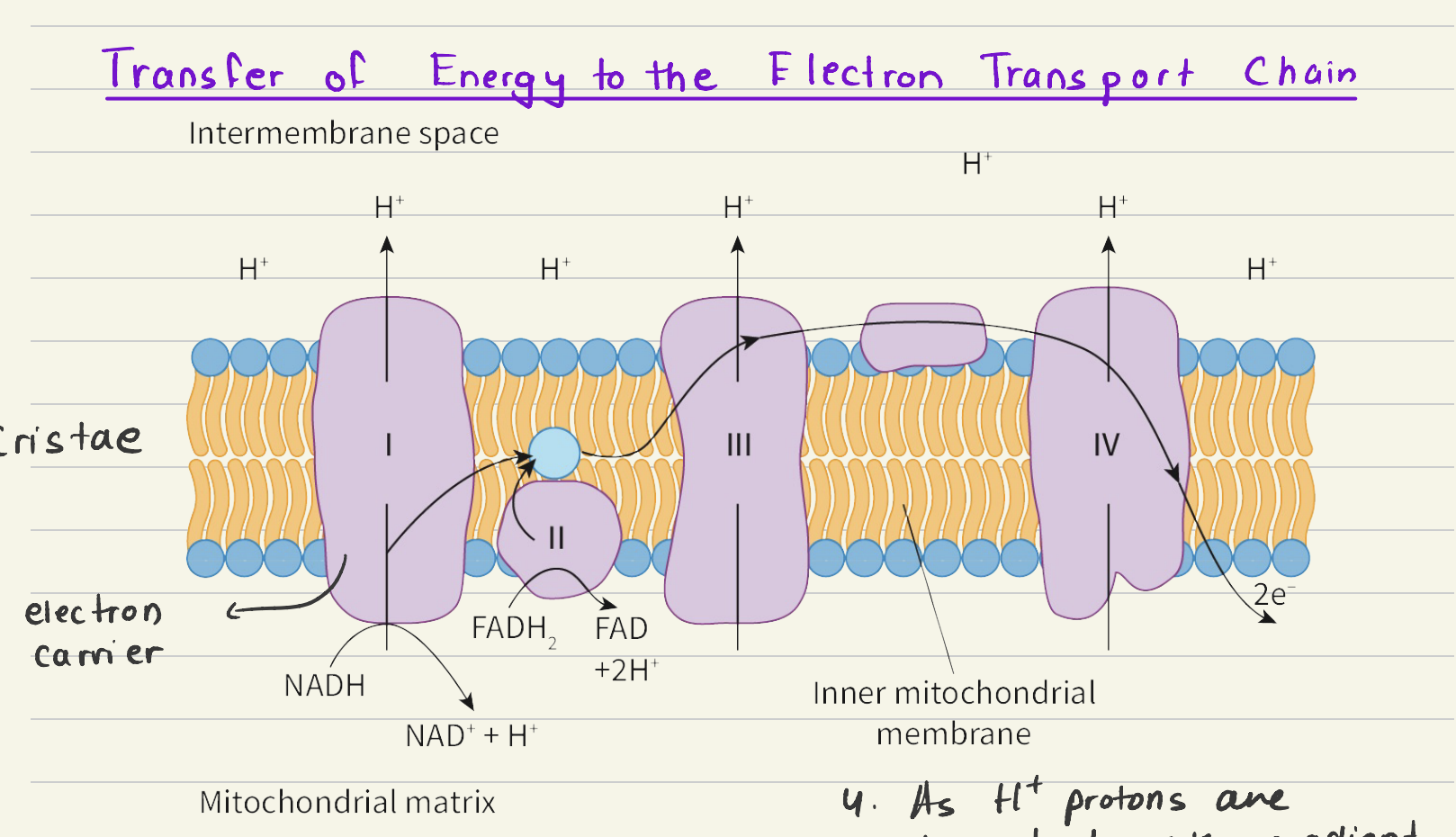
Electron transport chain:
NADH → NAD+ + H+ - Electrons passed onto the first electron carrier
Movement of electrons across carriers generates energy
Energy used to move H+ proton across the bilayer against the conc. gradient
FADH2 → FAD + 2H+
At the final electron carrier, oxygen accepts electrons and protons: 2H+ + 2e- + O → H2O
As H+ protons move down their conc. gradient via facilitated diffusion, energy is generated that ATP synthase uses to phosphorate: ADP + Pi → ATP + H2O
38 ATP per GLUCOSE molecule
Why is oxygen important as the final electron acceptor?
oxygen helps maintain the proton gradient
oxygen picks up de-energused electrons
so reduced FAD and NAD can be oxidised again
to provide protons for ATP Synthase
Chemiosmosis definiton
Role of Cristae
folded to increase SA:VOL for diffusion
contains ATP Synthase for synthesis of ATP
site of oxidative phosphorylation
site of electron transport chain
creates a proton gradient
Electron Transport Chain definition
-The addition of oxygen
-The removal of oxygen
-because they involve the transfer of electrons from one substance to another
-They often link oxidations and reductions in cells
-The main electron carrier in respiration is NAD
Name for types of anaerobic respiration:
yeast: fermentation
animal: glycolysis / lactate fermentation
Explain why ATP is synthesised in large amounts?
ATP cannot be stored
ATP only releases small amounts of energy at a time
ATP is required to drive many cellular process : active transport, synthesis of macromolecules