Lecture 9 - Antigen Processing and Presentation to T cells
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
What are the 2 major intracellular compartments of cells?
Cytosol
Vesicular System (ER, golgi, endosomes)
How do T cells respond to cytosolic pathogens? (Degradation, presentation, effect)
pathogens degraded in: cytosol
peptides bind to: MHC class I
presented to: CD8 T cells (cytotoxic)
effect on presenting cell: cell death
How do T cells respond to intravesicular pathogens? (Degradation, presentation, effect)
pathogens degraded in: endocytic vesicles
peptides bind to: MHC class II
presented to: CD4 T cells
effect on presenting cell: activation of macrophage, kills intravesicular bacteria and parasites
How do T cells respond to extracellular pathogens? (Degradation, presentation, effect)
pathogens degraded in: endocytic vesicles
peptides bind to: MHC class II
presented to: CD4 T cells
effect on presenting cell: activation of B cells to secrete antibodies
How does cross-presentation cause presentation of exogenous antigens on MHC I molecules?
specialized dendritic cells can cross present endocytosed/phagocytosed antigens on MHC I
cDC1 (Batf-3 dependent DCs) are better than cDC2 at cross presentation
How does cross presentation cause presentation of intracellular antigens on MHC class II?
autophagy - cytoplasmic contents are enclosed in membranes and degraded
contributes to CD4 cell tolerance to self antigens in thymus
What is the overview of MHC I peptide loading?
peptides are generated in the cytosol by the proteasome
then they are transported through TAP into the ER
then they are loaded on MHC I in ER and transported to the plasma membrane
What is the role of the proteasome?
it degrades ubiquitinated proteins to generate peptides for MHC-I
What is the structure of the proteasome
20S catalytic core with 2 alpha and 2 beta rings
19S cap that binds K48-linked ubiquitinated proteins
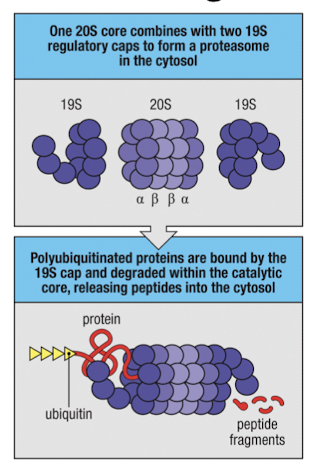
What is significant about the 19S cap on proteasomes?
it binds K48-linked ubiquitinated proteins and delivers them to the core for degredation
How is the proteasome altered during infections?
interferons β1i, β2i, and β5i are upregulated by interferons to create the immunoproteasome.
these replace the original β proteasome subunits, increasing cleavage after hydrophobic residues
IFN-γ induces the PA28 proteasome activator complex, replacing the 19s cap and making a conformational change
What is the role of the TAP transporter?
it translocates peptides into the endoplasmic reticulum
TAP1 + TAP2 = heterodimer that requires ATP
TAP prefers peptides of 8-16 amino acids w/ hydrophobic or basic C-termini (from immunoproteasome)
TAP1 and TAP2 are located in the MHC locus
What happens when mice and people lack TAP1 or TAP2?
they will have few MHC-I molecules on their surface
How does MHC-I stay stable in the absence of infection?
self peptides bind, because peptides are required for MHC-I stability
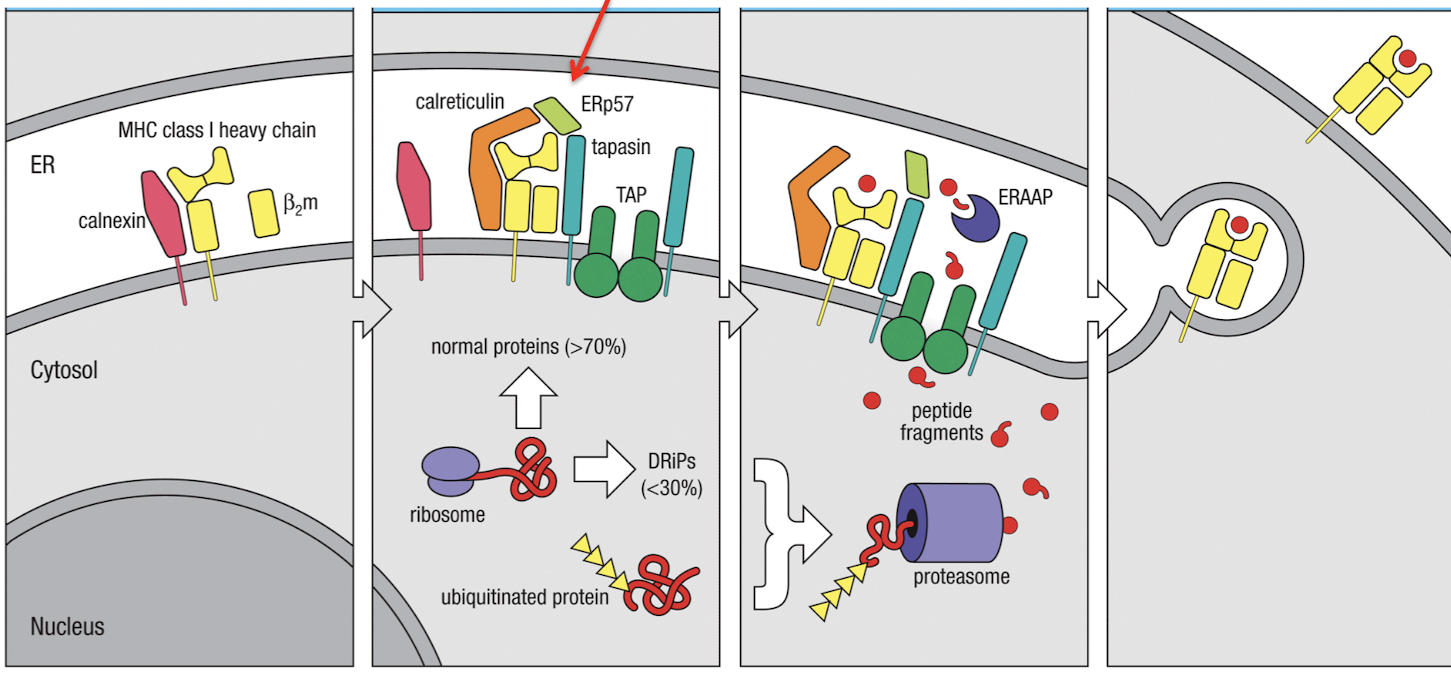
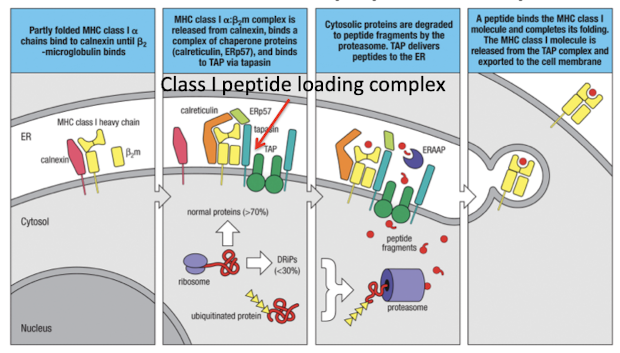
What are the 6 steps for the generation of MHC-I peptide complexes
MHC-I enters ER where it binds a calnexin chaperone
Calnexin is released and MHC binds other chaperones: calreticulin and Erp57
Tapasin binds MHC-I to TAP, making the Class I loading complex
peptides are transported and ERAAP cleaves amino terminus of peptide into 8-10 amino acids
peptide editing ensures sufficient affinity for MHC binding, then MHC-I is released and exported
if peptide dissociated from MHC-I on surface, MHC-Ia dissociates from b2m and is degraded
How do DNA viruses block MHC-I peptide processing and presentation?
viral evasins US6 and ICP7 block peptide movement through the TAP transporter
protein E19 competes with tapasin and inhibits peptide loading
mk3 protein targets MHC-I for degredation. bythe proteasome
ERAD (ER associated protein degredation) is co-opted by viruses to degrade MHC-I
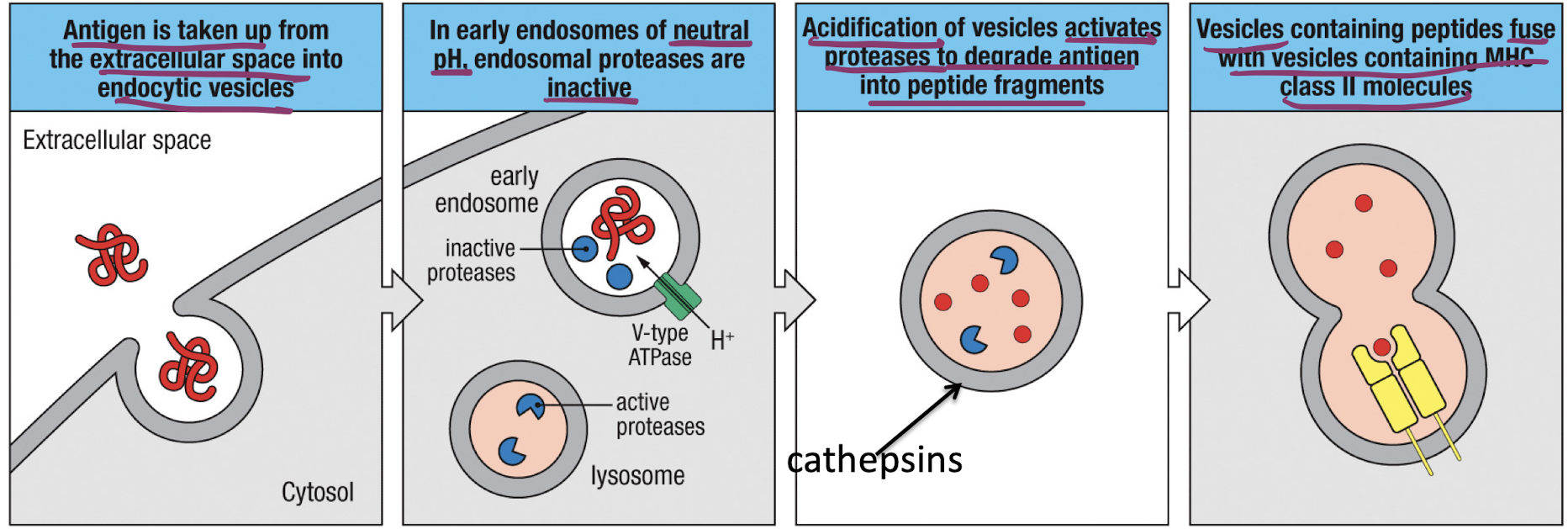
What is the overview of MHC II peptide loading?
Antigen uptake occurs due to B-cells, macrophages and dendritic cells, or macropinocytosis
V-type ATPase causes acidification of endosomes, activating proteases
vesicles containing MHC-II molecules fuse with acidified endosomes enabling peptide loasing on MHC-II
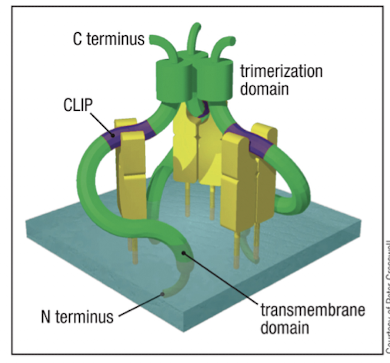
What is the role of the invariant chain (Ii) for MHC-II?
it directs newly synthesized MHC-II to vesicles and prevents it from binding peptides in the ER
delivers MHC-II to low pH endosomes where peptide loading occurs
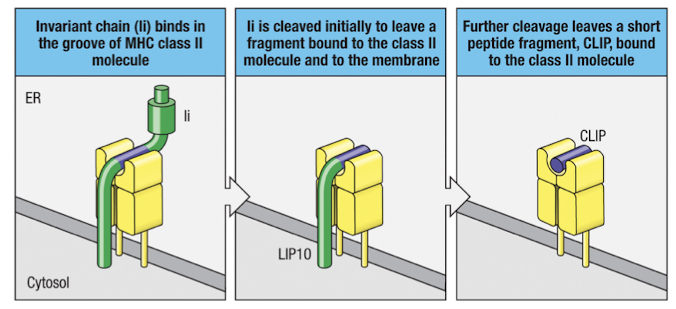
How is the invariant chain processed in acidified vesicles?
It is cleaved by proteases like cathespsin S to leave behind CLIP in the peptide binding domain, MHC-II:CLIP complex
What is the function of HLA-DM in MHC-II peptide loading?
HLA-DM catalyzes the exchange of CLIP for other peptides in vesicles
binds MHC-II to release CLIP, stabilizes empty MHC-II
induced by IFNγ
What is the function of HLA-DO?
HLA-DO competes with HLA-DM by binding to it and blocking its activity
expressed by TECs, B cells, and DCs
What is the function of MARCH-I?
it regulates MHCII turnover and processing of new peptides in resting vs activated dendritic cells; it also regulates CD86(a t cell co stimulatory molecule)
ubiquitinates MHCs in immature DCs, targeting them for degredation
activation of DCs stops transcription of MARCH-I
MHC gene structure
3 MHC-I genes and 3 MHC-II genes
3IFNS (alpha, beta, and gamma) increase transcription of MHC related molecules
IFNγ increases expression of MHC-II genes, Ii, HLAs (CIITA gene dependent)
What is significant about MHC genes?
They are highly polymorphic
what does it mean that MHC expression combines polymorphism and polygeny?
polymorphism = different alleles
polygeny = multiple genes
combines to for large diversity in MHC expression
What does it mean that T cell recognition is MHC restricted?
TCRs must contact both peptide and MHC residues (it must be compatible with both, not just one!)
Alloreactive T cells
t cells that are reactive to the MHC of somebody else
common (1-10% of t cells)
happens because CDR1 and CDR2 have inherent affinity for MHC
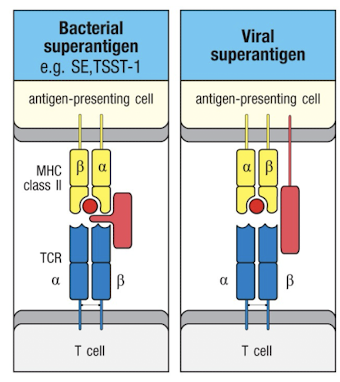
Superantigens
can activate t cells irrespective of TCR:pMHC specificity
binding of super antigens causes a massive cytokine release by t-cells