SNAB TOPIC 2- Genes and Health
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
2.1 i) Know the properties of gas exchange surfaces in living organisms
large surface area to volume ratio
minimised thickness of surface
maintained difference in concentration
2.1 ii) Understand how the rate of diffusion is dependent on these properties and
can be calculated using Fick's Law of Diffusion.
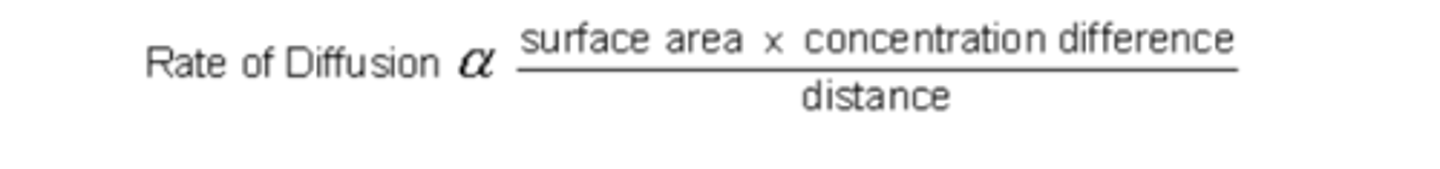
2.1 iii) Understand how the structure of the mammalian lung is adapted for rapid gaseous exchange.
Mammalian lungs:
-Large surface area to volume ratio, with many alveoli
-Minimised thickness of surface, ast the alveoli are just one cell thick.
-Maximised difference in concentration gradient, as blood with high CO2 and low O2 flows to the alveolar capillaries, maintaining the concentration gradient.
2.2 i) Know the structure and properties of cell membranes.
-Bilayer of phospholipids, phosphate heads facing to aqueous side, lipid tails facing inside the bilayer
-Embedded molecules (channel proteins, glycoproteins, glycolipids, carrier proteins, receptor proteins etc.)
-Gases or Small hydrophobic molecules can pass through rapidly
-Small polar molecules, e.g. water/ethanol, can pass (slowly)
-Charged/large polar/very large hydrophobic molecules cannot directly enter without protein assistance or without being broken down
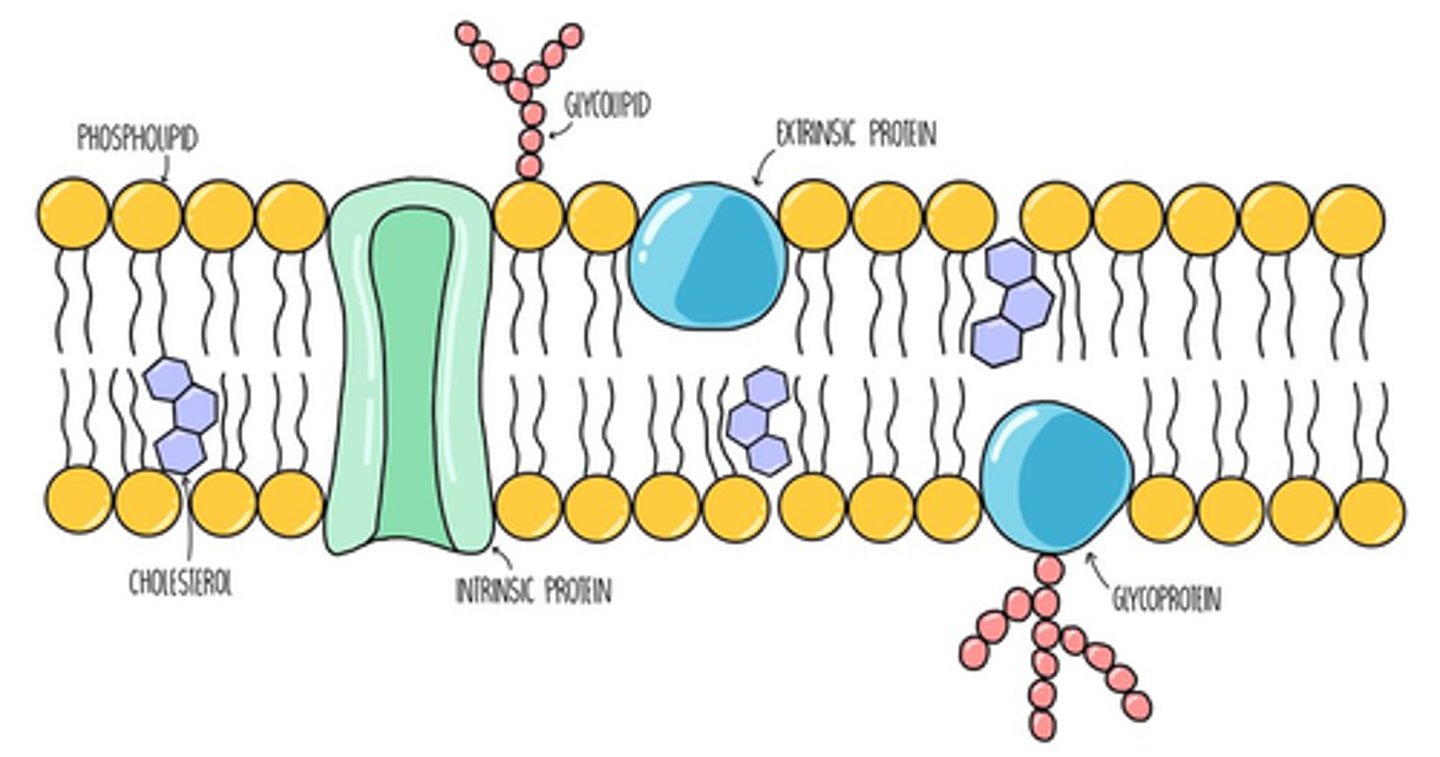
2.2 ii) Understand how models such as the fluid mosaic model of cell membranes are interpretations of data used to develop scientific explanations of the structure and properties of cell membranes.
-Original model: three layer protein lipid sandwich, from electron micrographs with dark outer layers (thought to be proteins) and lighter regions (thought to be the lipids). Model does not hold because phosphate heads do not face the water
-Two types of proteins, one could easily be separated with an ionic solution (peripheral proteins) and the others required more drastic action like adding detergents (integral proteins)
-Freeze fracture electron microscopy studies allowed for a micrograph of one of the layers. It revealed a smooth mosaic like surface, with larger particles interspersed (integral proteins)
CORE PRACTICAL 3:
Investigate membrane structure, including the effect of alcohol concentration or temperature on membrane permeability.
Method
1) Use cork borer & scalpel to cut equal length cylinders of beetroot on white tile
2) Wash and blot dry with filter paper
3) Fill boiling tubes with equal cm3 of distilled water
4) Place each boiling tube in different water baths at different temperatures
5) Add a cylinder of beetroot in each tube once it is at the right temperature
6)Set a timer for 30 minutes
7) Set a colorimeter to blue/green filter
8) Calibrate colorimeter with cuvette that has water
9) Once finished, add 2cm3 of beetroot solution in a new cuvette and measure % absorbance at different temperatures
Control variables
Volume of distilled water
Time left in water
Size of beetroot piece
Colorimeter used
Volume of beetroot solution
Control
Beetroot piece in 10cm3 of distilled water at room temp.
Graph: plot graph of %absorbance against temperature.
2.3 Understand what is meant by osmosis in terms of the movement of free water molecules through a partially permeable membrane (consideration of water potential is not required).
The movement of water from low to high solute concentration, through a partially permeable membrane
2.4 ii) Understand the involvement of carrier and channel proteins in membrane transport.
Carrier proteins enable active transport by undergoing conformational changes with the hydrophilic groups
Channel proteins enable facilitated diffusion by acting as a pore
2.4 i) Understand what is meant by passive transport (diffusion, facilitated
diffusion), active transport (including the role of ATP as an immediate source of energy), endocytosis and exocytosis.
Passive transport: substances moving down the concentration gradient
Active transport:
Movement of molecules through a carrier protein, typically against the concentration gradient, requiring energy in the form of ATP.
ATP releases energy when hydrolysed, and requires energy to be regenerated
Across a membrane
-Diffusion: the passive transport of small, non polar, lipid soluble molecules
-Facilitated diffusion: Movement through a channel protein
-Endocytosis/exocytosis: the transportation of large particles enclosed in vesicles that fuses with the cell membrane and are released on the other side. These are both active processes
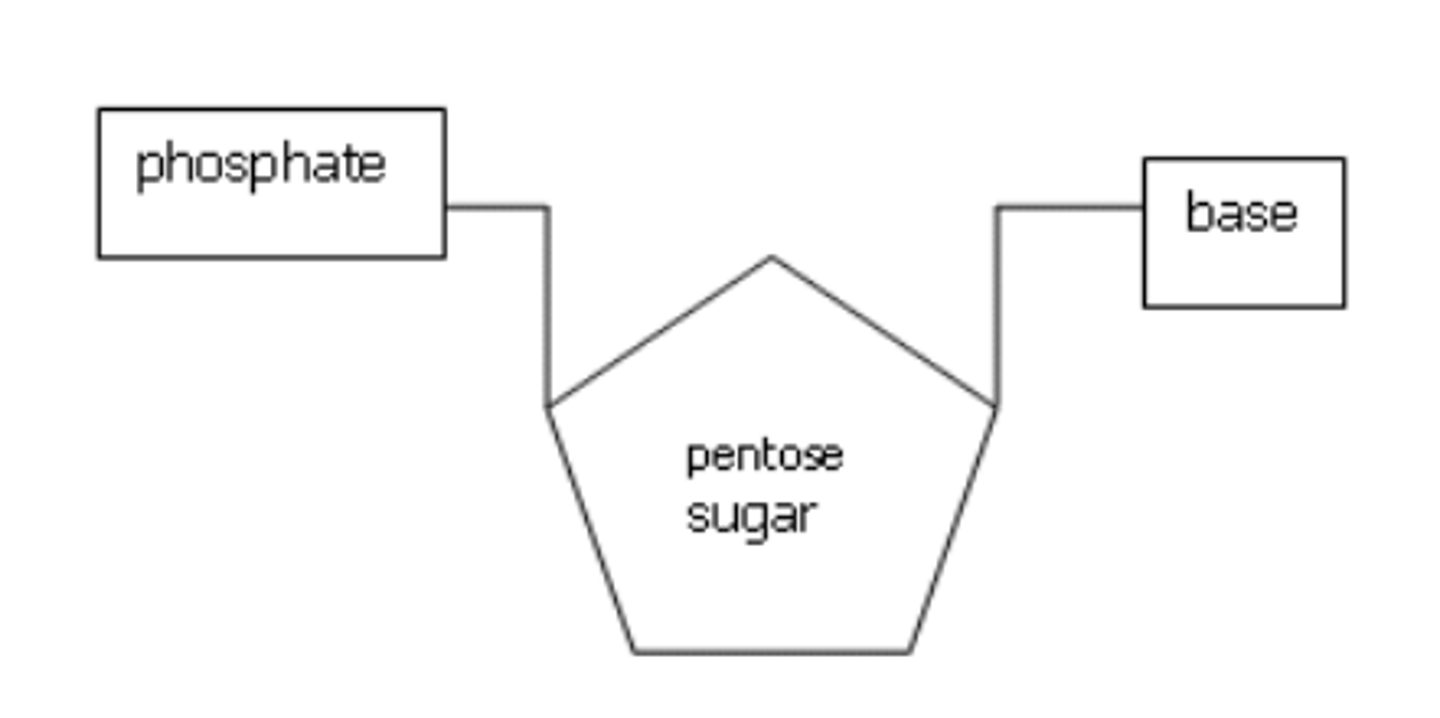
2.5 i) PART A Know the basic structure of mononucleotides
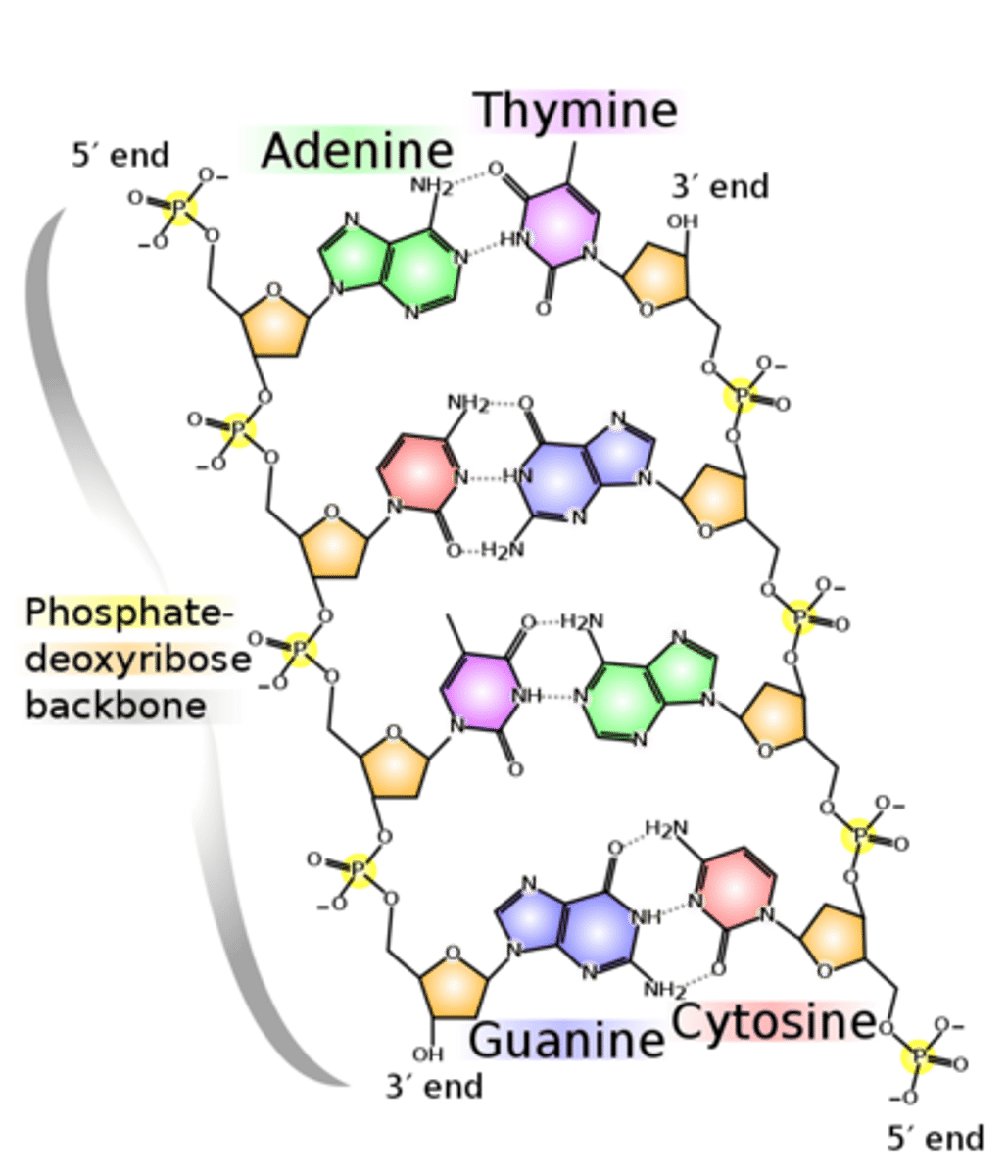
2.5 ii) Know how complementary base pairing and the hydrogen bonding between two complementary strands are involved in the formation of the DNA double helix.
Complementary base pairing and hydrogen bonding stabilize the DNA double helix shape
A=T (two hydrogen bonds)
G≡C (three hydrogen bonds)
2.5 ii) PART B Know the structures of DNA (polynucleotides composed of mononucleotides linked through condensation reactions).
Structure of DNA:
Double stranded, alpha double helix with sugar-phosphate backbone on each strand
Deoxyribose sugar
Nucleotide sequences are held together by phosphodiester bonds (between phosphate and carbon 5).
Two strands are held together by hydrogen bonds between bases
The two strands are alternate- one strand is from 5' to 3', while the other strand is from 3' to 5'
(referring to which carbon the phosphodiester bond is on)
2.5 ii) PART C Know the basic structure RNA (polynucleotides composed of mononucleotides linked through condensation reactions).
A,G (purine) C, U (pyrimidine) bases
Single stranded
Ribose sugar
Phosphodiester bonds
tRNA has an anticodon region and attachment site for an amino acid
2.6 i) (PART A- transcription) Understand the process of protein synthesis (transcription) including the role of RNA polymerase & messenger RNA
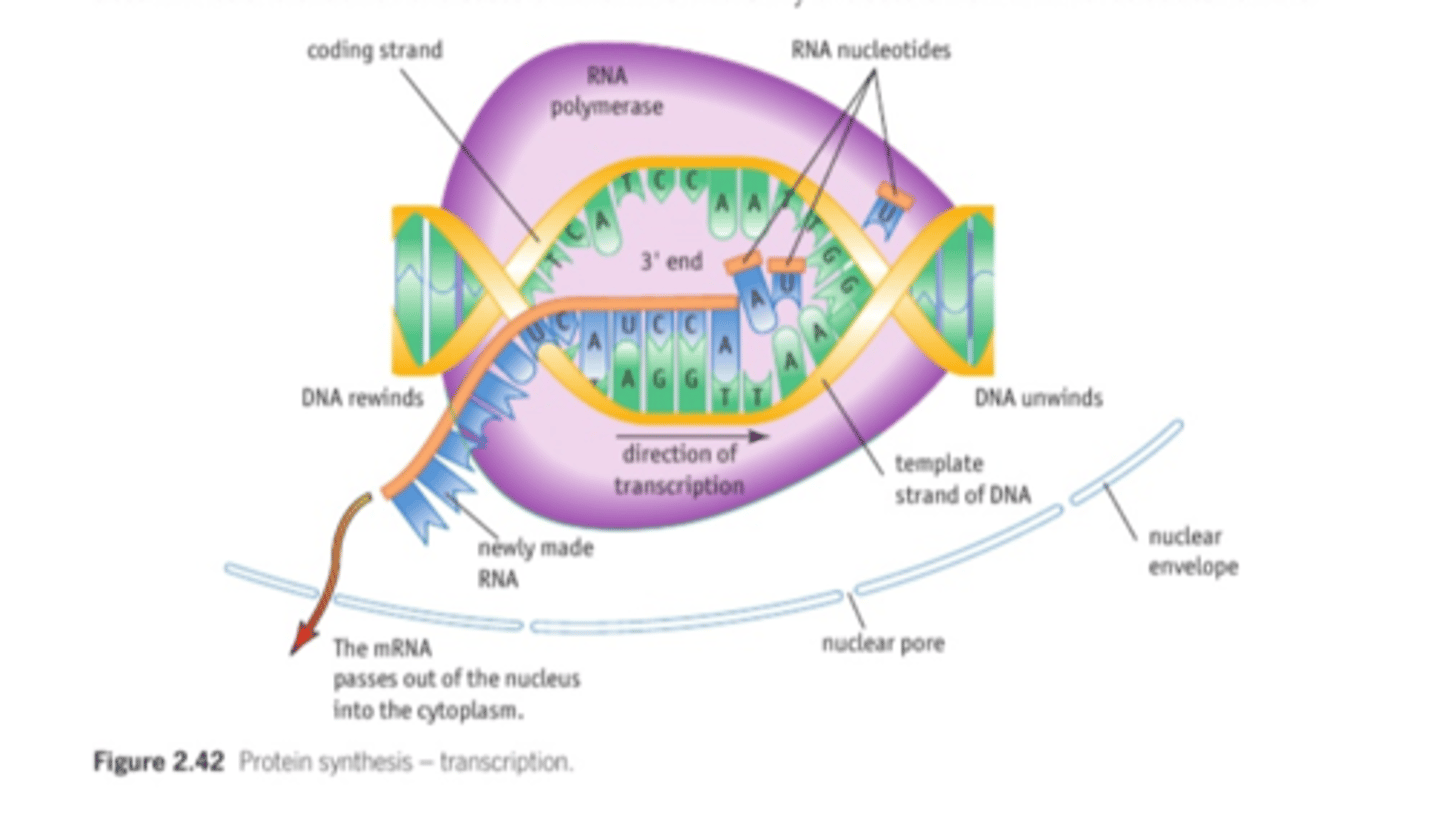
2.6 i) (PART B- translation) Understand the process of protein synthesis (transcription) including the role of RNA polymerase, translation, messenger RNA, transfer RNA, ribosomes and the role of start and stop codons.
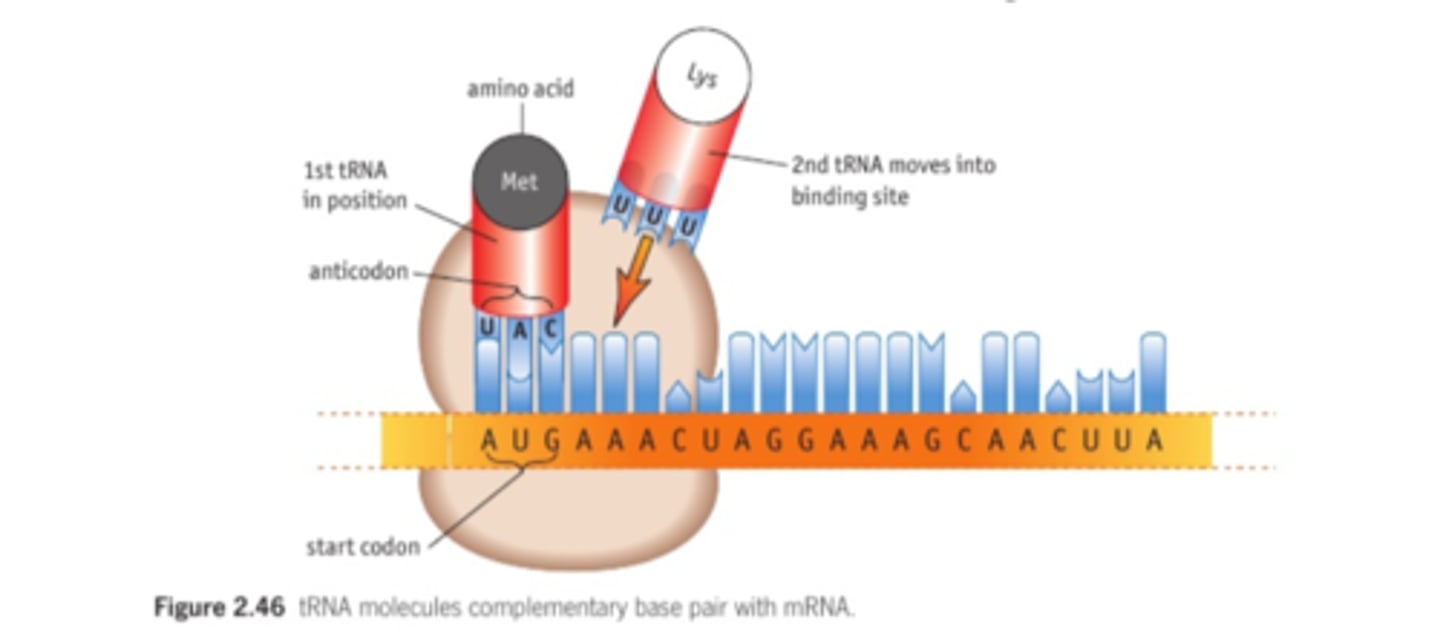
2.6 ii) Understand the roles of the DNA template (antisense) strand in
transcription, codons on messenger RNA and anticodons on transfer RNA.
The DNA template strand has the target gene. mRNA creates a complementary strand to this and leaves the nucleus.
Codons on mRNA and anticodons on tRNA are complementary, so will bind
2.7 Understand the nature of the genetic code
Triplet code
Non-overlapping- each nucleotide is part of only one codon, cannot be part of two adjacent codons
Degenerate- multiple codons coding for one amino acid
2.8 Know that a gene is a sequence of bases on a DNA molecule that codes for a sequence of amino acids in a polypeptide chain.
a gene is a sequence of bases on a DNA molecule that codes for a sequence of amino acids in a polypeptide chain.
2.9 i) Know the basic structure of an amino acid (structures of specific amino acids are not required).
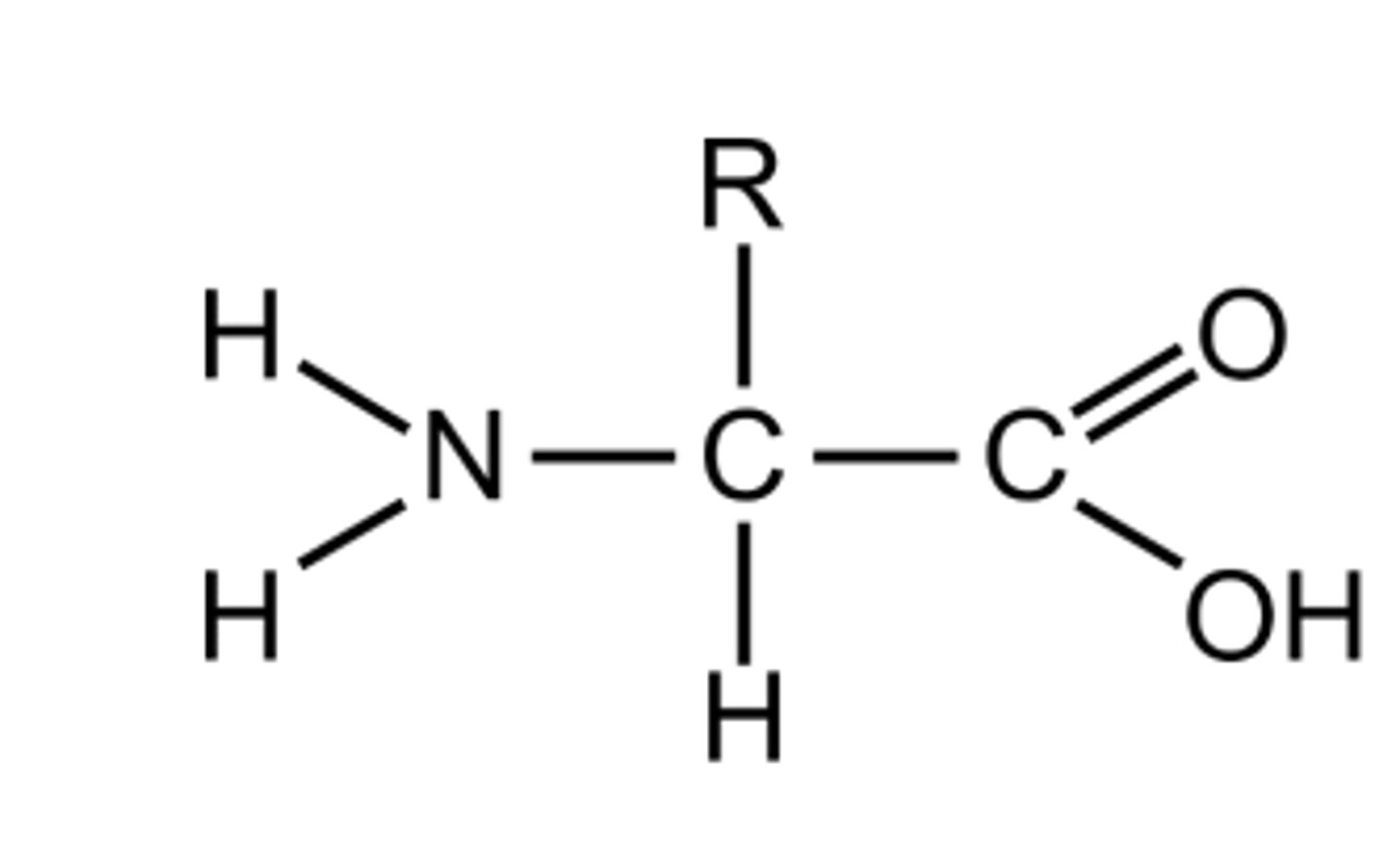
2.9 ii) Understand the formation of polypeptides and proteins
Amino acid monomers linked by peptide bonds in condensation reactions
2.9 iii) Understand the significance of a protein's primary structure in determining its three-dimensional structure and properties (globular and fibrous proteins and the types of bonds involved in its three-dimensional structure).
Primary structure: sequence of amino acids
protein formation:
•sequence of amino acids=primary structure
•secondary structure of proteins involve beta pleated sheets and alpha helices
•R groups interactions in the sequence of amino acids determines the 3d , tertiary shape---> disulfide bridges, hydrogen bonds
this happens in the ER and the protein is then modified in the golgi apparatus
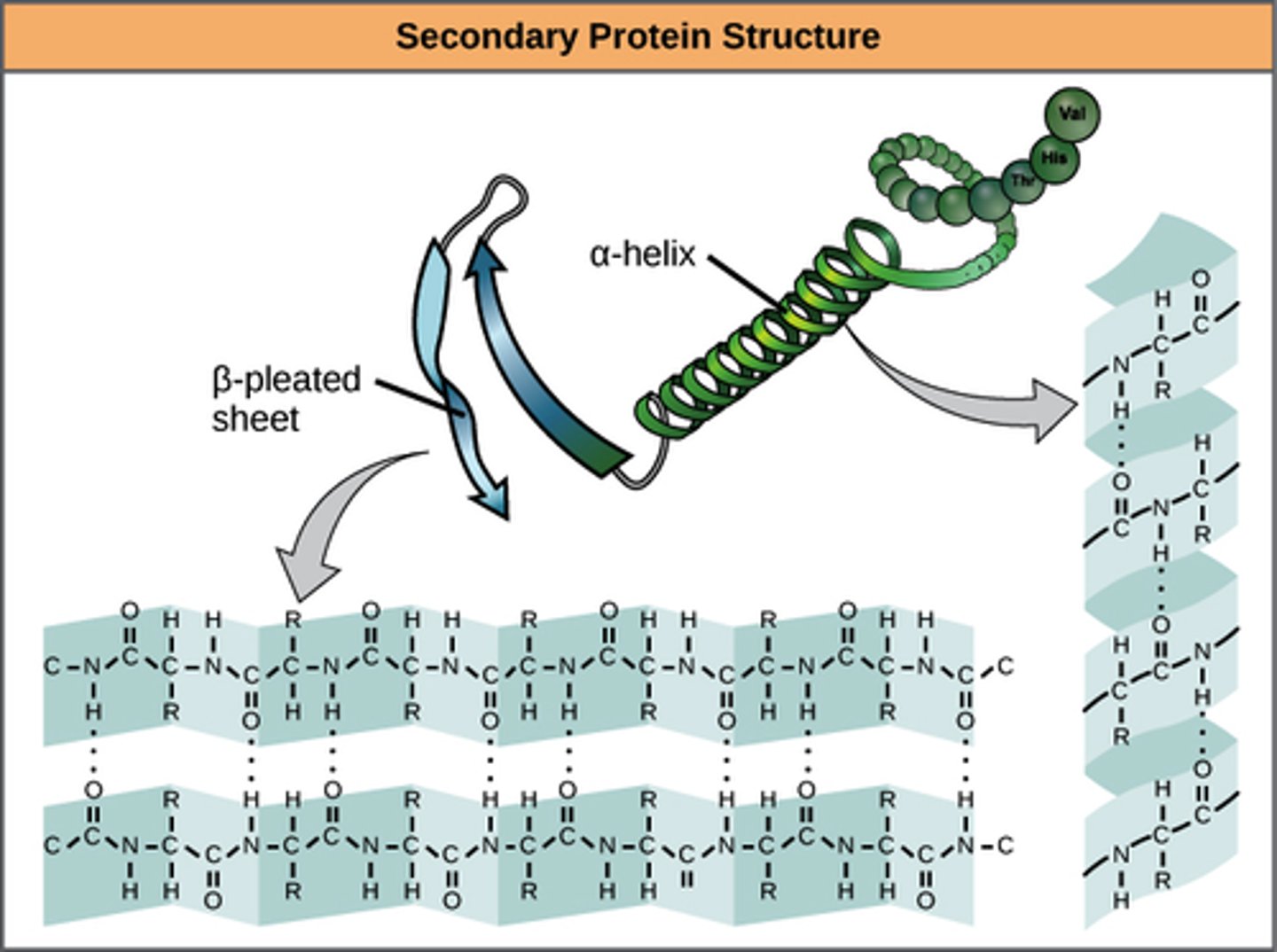
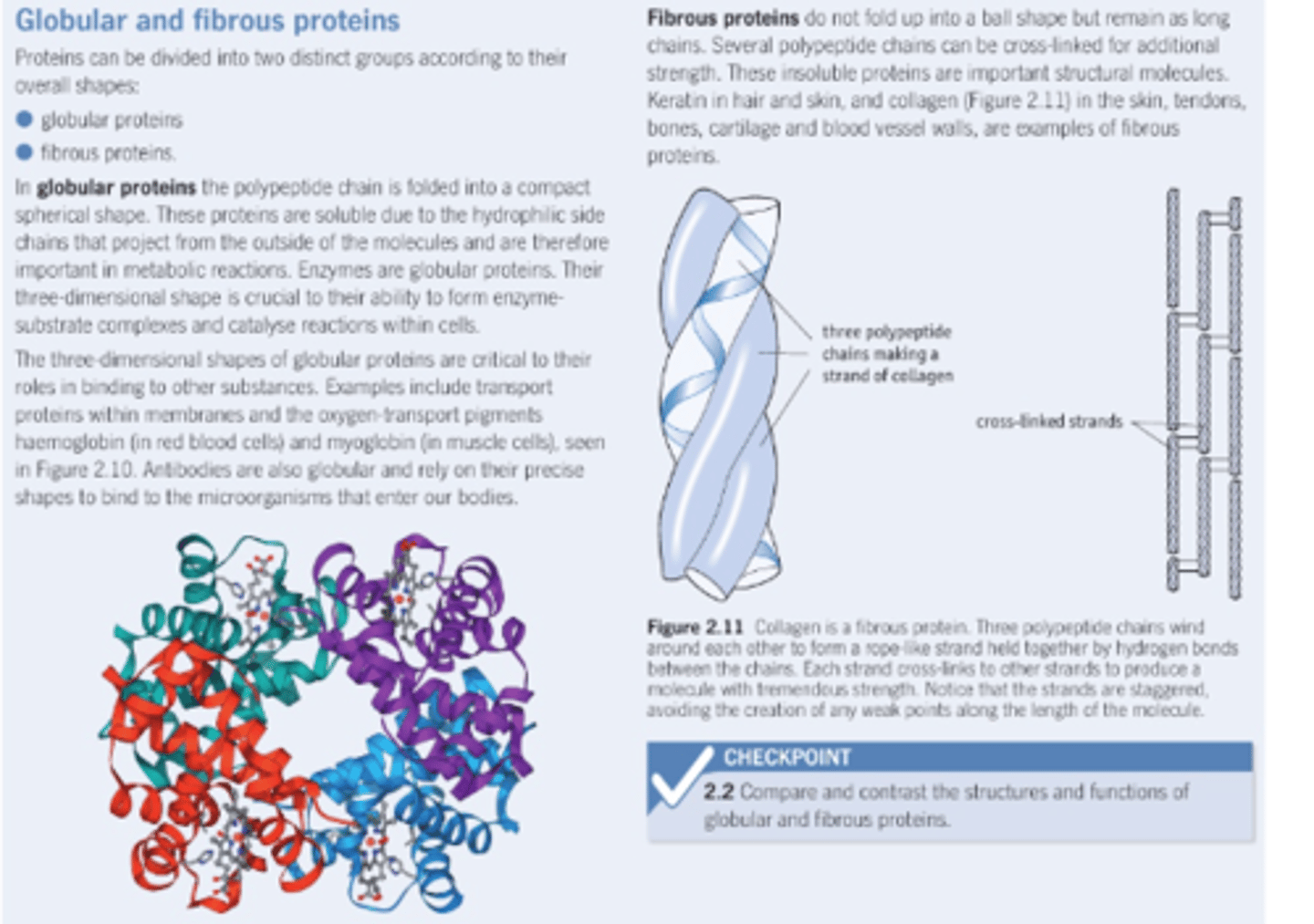
2.9 iv) Know the molecular structure of a globular protein and a fibrous protein and understand how their structures relate to their functions (including haemoglobin and collagen).
Globular protein:
Compact spherical shape, hydrophilic side chains on outside mean they are soluble.
-Unique 3D shape enables specific active site for enzymes
Fibrous protein
Insoluble protein, long polypeptide chains cross linked for additional strength. Held together by hydrogen bonds.
-Cross linking of chains & winding structure of strands allows structural strength in proteins like collagen.
Typically no tertiary structure as most fibrous proteins only have one secondary structure
2.10 i) Understand the mechanism of action and the specificity of enzymes in terms of their three-dimensional structure.
Lock and key (enzyme specific for reaction), induced fit (flexible active site)
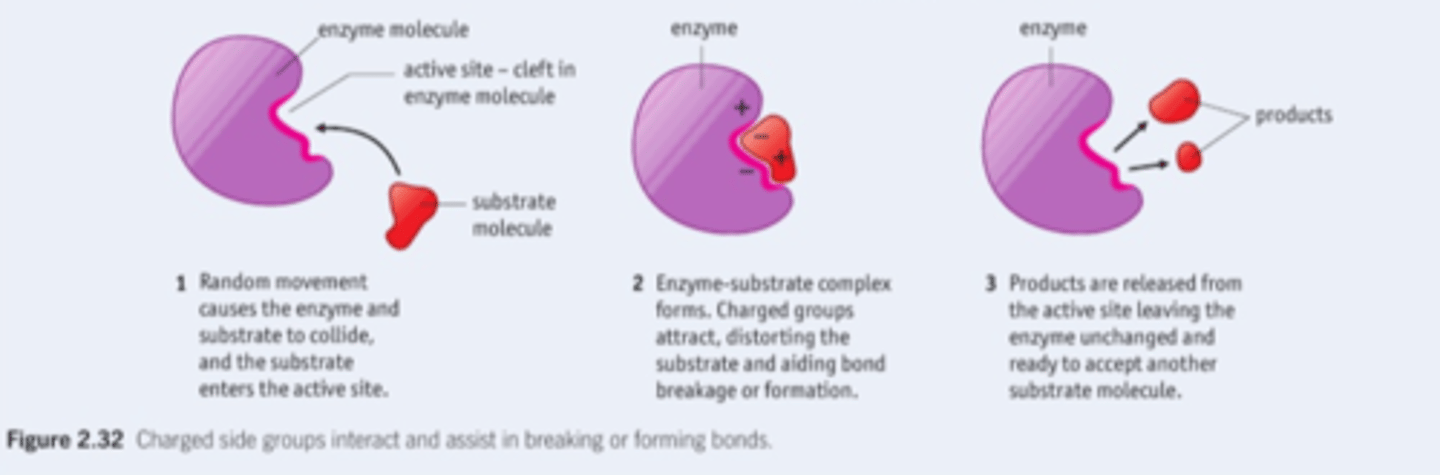
2.10 ii) Understand that enzymes are biological catalysts that reduce activation energy.
enzymes are biological catalysts that reduce activation energy.
CORE PRACTICAL 4:
Investigate the effect of enzyme and substrate concentrations on the initial
rates of reactions.
Vary substrate/enzyme concentration, keep the other conc. controlled.
Control variables
Temperature
Volume of enzyme/substrate solution
Equipment
Range of concentrations for enzyme(trypsin, we did catalase) /substrate(casein, we did h2o2)
Thermometer
Water bath
Test tubes
Stop watch
Colorimeter OR gas syringe/inverted burette
Control
0% enzyme/substrate solution can be used and added to its component. The results can be compared to the control
Method
Keep temp. constant. Add substrate to varying enzyme concentrations and measure the absorbance of samples in the colorimeter, or measure the volume of gas produced. Do this at regular intervals using a stopwatch
Plot a graph of each reaction, with absorbance/volume of gas released against time.
The gradient of the tangent to the initial point will give the initial rate of reaction for each curve.
Plot a second graph of the rates of reaction (y axis) against the enzyme/substrate concentration (on x axis)
Calc
Rate = 1/time
Mean values to be calculated using repeat data
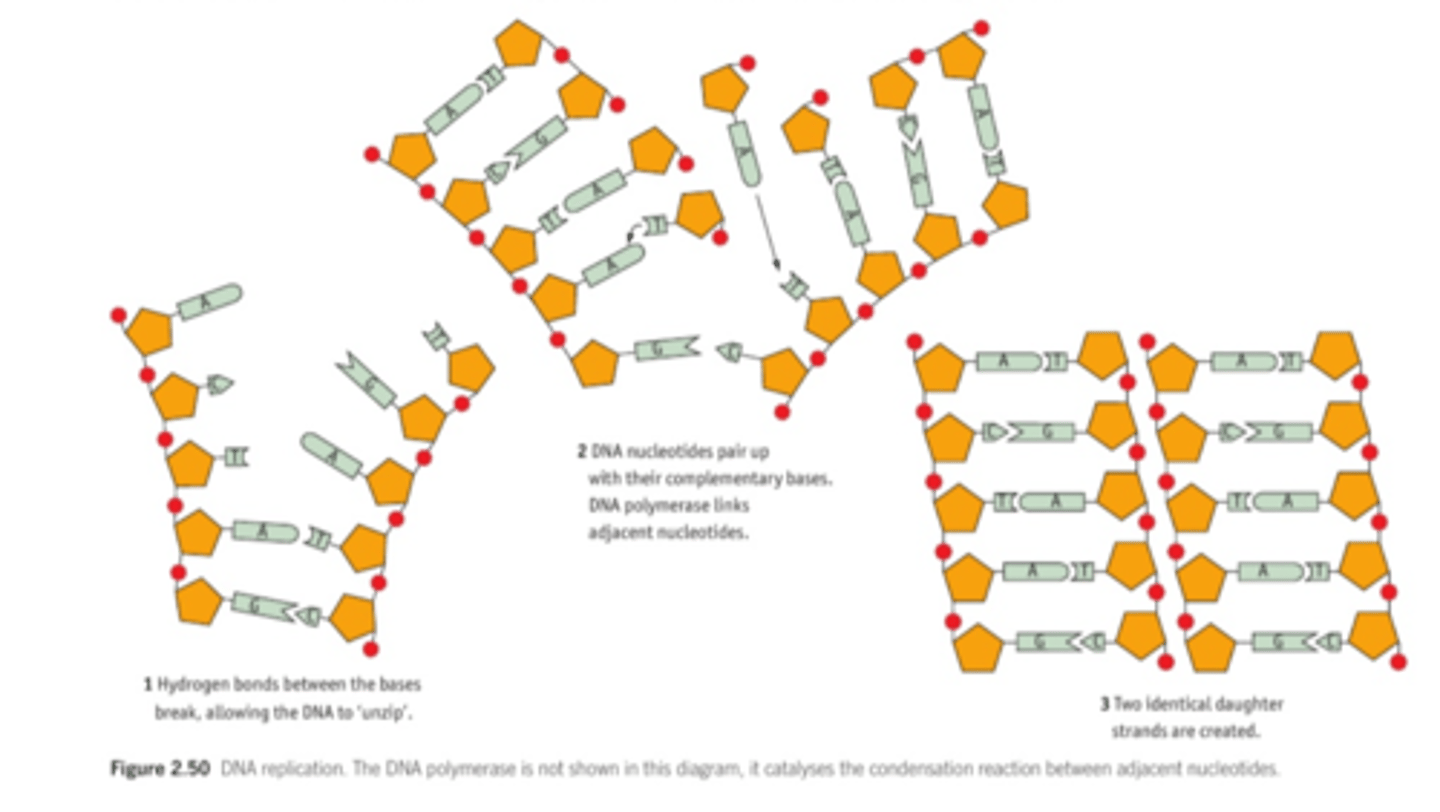
2.11 i) Understand the process of DNA replication, including the role of DNA polymerase.
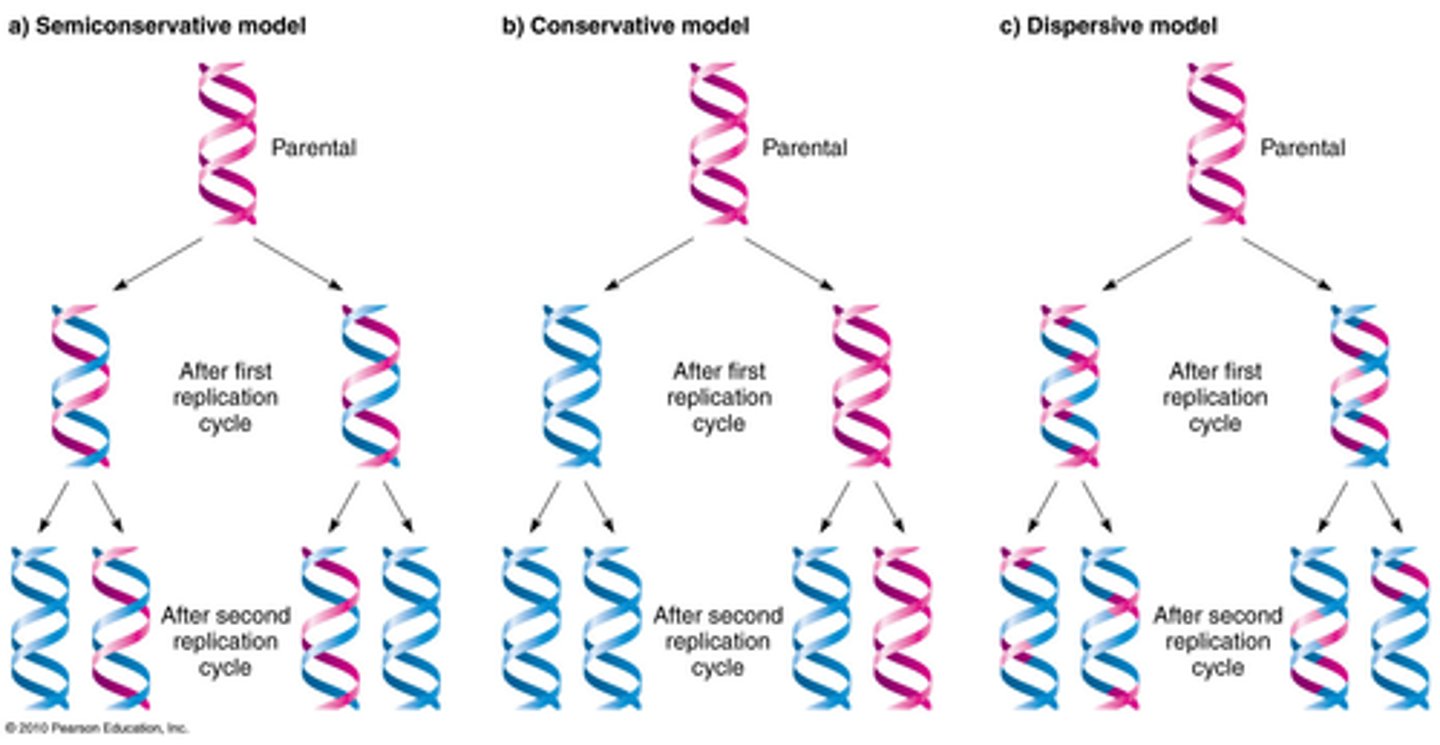
2.11 ii) Understand how Meselson and Stahl's classic experiment provided new data that supported the accepted theory of replication of DNA and refuted competing theories.
Meselson and Stahl's experiment:
-Bacteria grown in heavy nitrogen isotope (N15)
[DNA strands=heavy:heavy]
-Bacteria is transferred to lighter nitrogen isotope (N14)
-After one generation, medium (weight) DNA was observed
[DNA strands= heavy:light]
-After another generation, another band appeared that was entirely light (N14)
[DNA strands= light:light]
-this refuted the conservative and fragmentary replication models
-it supported the semi conservative model
![<p><span style="text-decoration:underline">Meselson and Stahl's experiment:</span> </p><p>-Bacteria grown in heavy nitrogen isotope (N15) </p><p>[DNA strands=heavy:heavy] </p><p></p><p>-Bacteria is transferred to lighter nitrogen isotope (N14)</p><p>-After one generation, medium (weight) DNA was observed</p><p>[DNA strands= heavy:light]</p><p></p><p>-After another generation, another band appeared that was entirely light (N14)</p><p>[DNA strands= light:light]</p><p></p><p>-this refuted the conservative and fragmentary replication models</p><p>-it supported the semi conservative model</p>](https://knowt-user-attachments.s3.amazonaws.com/74ef9318-611b-473f-b06b-80cad0e4ee25.png)
Models of DNA Replication
2.12 i) Understand how errors in DNA replication can give rise to mutations.
Substitution, Insertion, Deletion, Duplication, Inversion.
Mutations change the base sequence in mRNA which means the protein's structure is changed
A frameshift mutation is an example of when nucleotides are either deleted or inserted, altering the entire sequence of bases and therefore amino acids that are produced in translation.
2.12 ii) Understand how cystic fibrosis results from one of a number of possible gene mutations.
Hundreds of different mutations affect the CFTR channel and its functionality differently--> severity of symptoms varies.
(most common CF mutation is a deletion of 3 nucleotides)
How does the CFTR protein work to ensure the mucus is the right consistency?
Marking points available
•Chloride ions leave cells (through the CFTR channel protein)
•sodium ions leave the cells (following the chloride ions)
•This increases the solute concentration in the mucus
•(because of this) water moves out of the cells by osmosis, and into the mucus
2.13 i) Know the meaning of the terms: gene, allele, genotype, phenotype,
recessive, dominant, incomplete dominance, homozygote and heterozygote.
Gene- a sequence of bases that codes for a protein
Allele- a variation/form of a gene
Genotype-all the alleles of an organism
Phenotype- the set of observable characteristics of an individual resulting from the interaction of its genotype with the environment
Recessive- an allele that is only expressed if two copies are present
Dominant- An allele that is expressed even if only one copy of the allele is present
Incomplete dominance- where the dominant allele does not completely mask the effects of a recessive allele
Homozygote- An individual having two identical alleles of a gene
Heterozygote- an individual having two different alleles of a gene
2.13 ii) Understand patterns of inheritance, including the interpretation of genetic pedigree diagrams, in the context of monohybrid inheritance.
Monohybrid inheritance: the inheritance of a single characteristic controlled by different alleles
2.14 Understand how the expression of a gene mutation in people with cystic fibrosis impairs the functioning of the gaseous exchange, digestive and reproductive systems.
Respiratory system
-Build up of sticky mucus traps bacteria, increasing risk of infection
-Build up of mucus decreases the surface area available for gas exchange by blocking off alveoli
Reproductive system
-Cervical mucus blocks sperm from reaching the egg
-Sperm duct is blocked with mucus, sperm cannot leave testes
Digestive system
-Pancreatic duct becomes blocked with mucus so enzymes do not reach the small intestines, so fewer nutrients are absorbed
-Duodenum mucus lining becomes thick, reducing absorption of nutrients.
-Mucus can cause cysts to form in the pancreas, damaging insulin producing cells and leading to diabetes
2.15 i) Understand the uses of genetic screening, including the identification of carriers, pre-implantation genetic diagnosis (PGD) and prenatal testing, including amniocentesis and chorionic villus sampling.
PGD
Embryos created through IVF are tested for genetic disorders before being implanted
Prenatal testing
Amniocentesis: 14-16 weeks, tests foetal cells found in the amniotic fluid
Chronic villus sampling:8-12 weeks, placenta tissue analysed. quicker than amniocentesis.
2.15 ii) Understand the implications of prenatal genetic screening.
Risk of harm to foetus
Risk of miscarriage
Outcome of testing may lead to an abortion
2.16 Be able to identify and discuss the social and ethical issues related to genetic screening from a range of ethical viewpoints.
-The cost of bringing up a baby with a genetic disorder
-The outcome of testing might lead to an abortion (right to life)
-Risk of harm to foetus
-Risk of miscarriage
-May cause distress to individuals
-May give information to family members who do not wish to know
-May result in difficulty finding a partner