catalysts
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
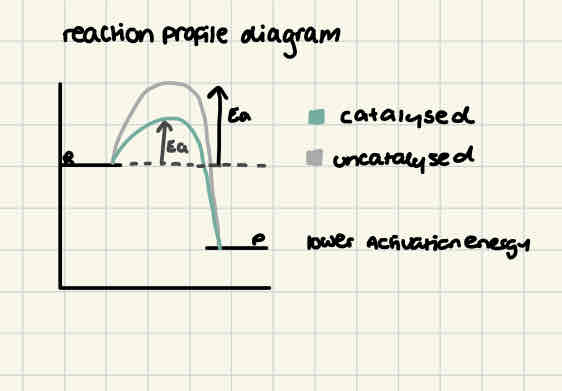
role of catalysts
increase the rate of reaction without being used up by providing an alternative route for a reaction with a lower activation energy requirement
reaction profile diagram for uncatalysed vs catalysed reaction
hetereogenous catalyst
a catalyst in a different phase/state from the reactants
how do hetereogenous catalysts work
Adsorption of the reactants at active sites on the surface may lead to catalytic action
the active site is the place where the reactants adsorb onto the surface of the catalyst, where bonds are formed between the reactant and surface of the catalyst
the reaction occurs on the surface
the products are desorbed from the surface