Biotechnology chapter 5-6
0.0(0)
Card Sorting
1/139
Earn XP
Description and Tags
Last updated 6:00 PM on 12/27/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
140 Terms
1
New cards
__*Describe some of the ways proteins are used in biotechnology products.*__
* Whole protein molecules such as recombinant insulin
* Contain protein molecules as a key ingredient such as enzymes in contact cleaners
* Contain **parts** of protein molecules such as the artificial sweetener Aspartame
* Whole organism are characterized as the **make new/novel proteins**
* Some products are used to study/synthesize proteins
* Contain protein molecules as a key ingredient such as enzymes in contact cleaners
* Contain **parts** of protein molecules such as the artificial sweetener Aspartame
* Whole organism are characterized as the **make new/novel proteins**
* Some products are used to study/synthesize proteins
2
New cards
3
New cards
* __*What must researchers do prior to producing a protein product?*__
* Learn about the structure/function of the protein/amino acid sequence
* **Ex:** molecular mass of a protein molecule achieved through using an instrument known as mass spectrometer
* **Ex:** molecular mass of a protein molecule achieved through using an instrument known as mass spectrometer
4
New cards
__*What else is important to researchers to know about proteins?*__
* 3D structure of a protein through the use of a x-ray crystallography and computer analysis of the x-ray diffraction dara
5
New cards
* __*How does this specific process of X-ray crystallography occur?*__
1. x-ray beam is shined on a very pure crystal on the protein of interest
2. once beam bits the atoms of a protein molecule in the crystal, the x-ray light is diffracted off the atoms
3. a detector then records the patter of the x-ray diffracted light
4. a trained technician with the aid of computers can interpret the x-ray diffraced data and create a 3D image of the protein molecule
6
New cards
* __*What are protein molecules?*__
* Polymers composed of amino acids
7
New cards
* __*What are some amino acids characteristics?*__
* Relatively small molecules
* **Central carbon+carboxyl group(COOH)** on one side and an **amino group(NH^2)** in the other side along with an **R group** that distinguishes it from other amino acids
* **Central carbon+carboxyl group(COOH)** on one side and an **amino group(NH^2)** in the other side along with an **R group** that distinguishes it from other amino acids
8
New cards
* __*What is the R group’s relative location?*__
* Attached at the central carbon and varies in length shape
9
New cards
* __*What does the R group indicate?*__
* In a formula it indicates a nonspecified side chain
* Also determines an amino acid’s interaction with other amino acids in a protein chain
* Also determines an amino acid’s interaction with other amino acids in a protein chain
10
New cards
* __*R-groups characteristics*__
* Charged (+ or -)
* polar( water soluble)
* nonpolar (not water soluble)
* polar( water soluble)
* nonpolar (not water soluble)
11
New cards
* __*What are proteins made up of?*__
* Contain tens or hundreds of amino acids chained together by peptide bonds
12
New cards
* __*Where is the peptide bond formed in proteins?*__
* Formed between carboxyl group of one amino acid and the amino group of an adjacent one
13
New cards
* __*Where does the bonding of amino acids through peptide bonds into long polypeptide molecules happen?*__
* Occurs in the cell’s ribosomes
14
New cards
* __*What is a polypeptide chain referred to as?*__
* Protein’s primary structure
15
New cards
* __*What does the messenger ribonucleic acid(mRNA) do?*__
* Located on one or more genes on the cell’s chromosomes that details which amino acids are to be placed into the polypeptide chain
16
New cards
* __*Give a brief summary of protein synthesis.*__
* Polypeptide chain: **Primary Structure**
* **Secondary Structure**
* In the polypeptide chain hydrogen bonding between hydrogen,oxegyn, and nitrogen atoms results in helices known as alpha helix and fold beta sheets
* **Tertiary Structure**
* More folding occurs due to the presence of charged/uncharged R-groups
* Example: amino acids with charges are attracted by amino acids of an opposite charge and are repelled by those of the same charge
* Section of a strand are pulled or pushed as these charged amino acids try to get closer to or farther from each other
* Ex: + charged arginine are attracted to - charged aspartic acid molecules
* Interactions between polar and nonpolar amino acids are also part of this structure as folding occurs when nonpolar amino acids are crowded together while polar amino acids attract other polar amino acids and repel nonpolar ones
* **Quarternary structure**
* Finally, disulfide bonds which occur between cysteine molecules produce and stabilizing tertiary folding in and between polypeptide chains
* Other bonds such as hydrogen bonds also help hold together multiple polypeptide chains while the disulfide bonds help also hold together individual polypeptide chains forming large loops
* **Secondary Structure**
* In the polypeptide chain hydrogen bonding between hydrogen,oxegyn, and nitrogen atoms results in helices known as alpha helix and fold beta sheets
* **Tertiary Structure**
* More folding occurs due to the presence of charged/uncharged R-groups
* Example: amino acids with charges are attracted by amino acids of an opposite charge and are repelled by those of the same charge
* Section of a strand are pulled or pushed as these charged amino acids try to get closer to or farther from each other
* Ex: + charged arginine are attracted to - charged aspartic acid molecules
* Interactions between polar and nonpolar amino acids are also part of this structure as folding occurs when nonpolar amino acids are crowded together while polar amino acids attract other polar amino acids and repel nonpolar ones
* **Quarternary structure**
* Finally, disulfide bonds which occur between cysteine molecules produce and stabilizing tertiary folding in and between polypeptide chains
* Other bonds such as hydrogen bonds also help hold together multiple polypeptide chains while the disulfide bonds help also hold together individual polypeptide chains forming large loops
17
New cards
* What do proteins demonstrate?
* Proteins demonstrate the relationship between structure and function
18
New cards
* __*Give one example:*__
* Glycoprotein(viral recognition protein) is a protein that has added sugar groups
* Glycoprotein 120 exists on the surface of HIV(the virus that leads to AIDS)
* Glycoprotein 120 exists on the surface of HIV(the virus that leads to AIDS)
19
New cards
* __*Further Explain what glycoprotein does:*__
* The HIV particle is recognizes,attaches, and infects a helper T-helper cell while the gp120 structure must be a precise shape and must perfectly match its human cell membrane receptors as it functions as a protein coat that asurrons the HIV’s genetic information found inside
20
New cards
* __*What is Glycoprotein structure?*__
* Single polypeptide chain build of hundreds of amino acids folded into 5 looped domains formed due to the disulfide bonds that stabilize shape of the functional protein
* The chains are highly glycosylated(bound with sugar groups) projecting out from the amino acids at certain intervals and act as recognition sites
* These regions match protein receptors on CD4 cells that HIV infects along with antibodies
* One of the looped domains has a shape that matches the CD4 molecules that is a recognition protein on the surface of white blood cells
* When the gp120 bumps into a CD4 molecule it triggers a set of reactions that results in the HIV particle being taken up by the cell
* This is how HIV begins to infect the cells
* The chains are highly glycosylated(bound with sugar groups) projecting out from the amino acids at certain intervals and act as recognition sites
* These regions match protein receptors on CD4 cells that HIV infects along with antibodies
* One of the looped domains has a shape that matches the CD4 molecules that is a recognition protein on the surface of white blood cells
* When the gp120 bumps into a CD4 molecule it triggers a set of reactions that results in the HIV particle being taken up by the cell
* This is how HIV begins to infect the cells
21
New cards
* __*What is HIV and how does it work?*__
* HIV is a retrovirus that has two molecules of RNA(gp120 polpeptide is coded on this RNA) along with two reverse transcriptase enzymes allowing it to create viral DNA
* When HIV infects a cell the RNA is reverse-transcribed into a DNA molecule with the reverse transcriptase enzymes
* The resulting”viral DNA” is then incorporated into the infected cell’s chromosomes and beings directing viral protein production
* To add on, the gp120 gene is read as well as produced leading to gp120 protein production for more viruses
* When HIV infects a cell the RNA is reverse-transcribed into a DNA molecule with the reverse transcriptase enzymes
* The resulting”viral DNA” is then incorporated into the infected cell’s chromosomes and beings directing viral protein production
* To add on, the gp120 gene is read as well as produced leading to gp120 protein production for more viruses
22
New cards
* __*Reverse Transcriptase*__
* Inaccurate enzyme that makes many mistakes upon transcription
* Results in variations in gp120’s looped domains leading to differences in the gp120 amino acid sequences
* This all causes HIV viral DNA to create new strands of HIV that certain antibodies may not reognize making it hard for companies to make a vaccine for this illness
* Results in variations in gp120’s looped domains leading to differences in the gp120 amino acid sequences
* This all causes HIV viral DNA to create new strands of HIV that certain antibodies may not reognize making it hard for companies to make a vaccine for this illness
23
New cards
* __*What is the function of an antibody?*__
* Recognize and bind foreign proteins or antigens for removal from the body
24
New cards
* __*Why are there different types of antibodies?*__
* Cells can recognize different types of illnesses
25
New cards
* __*What is the structure of antibodies?*__
* Four polypeptide chains(quaternary structure) attached through disulfide bonds
* Chains are arranged into a shape that resembles a Y shape
* Chains are arranged into a shape that resembles a Y shape
26
New cards
* __*What are the four polypeptide chains?*__
* Two heavy chains and two light chains
27
New cards
* __*What does the base of each antibody have?*__
* Identical primary sequence of amino acids
* Also called “the constant region”
* Also called “the constant region”
28
New cards
* __*Where is the variability seen in antibodies found?*__
* Found at the top of the Y in the variable region
29
New cards
* __*What is so unique about the DNA code for the primary sequence of this region?*__
* The DNA is shuffled to produce an infinite number of DNA codes thus creating an infinite number of amino acid sequences that produce thousands of different recognition sites for the ends of antibodies
30
New cards
* __*What are most antibodies?*__
* Very specific and only bonds to distinct molecules/specific regions known as epitopes
* In the lab, antibodies may be used to recognize and bind certain molecules
* This is useful as one can use this antibody specify to purify proteins from certain cell cultures
* In the lab, antibodies may be used to recognize and bind certain molecules
* This is useful as one can use this antibody specify to purify proteins from certain cell cultures
31
New cards
* __*How can a single protein be separated from other proteins?*__
* Antibody chromatography column
* In one column beads with antibodies are used as means of separating a target molecule from a mixture
* In more detail, the antibodies attach only to a matched molecule allowing all other unwanted molecules to pass through the column
* In one column beads with antibodies are used as means of separating a target molecule from a mixture
* In more detail, the antibodies attach only to a matched molecule allowing all other unwanted molecules to pass through the column
32
New cards
* __*What is ELISA?*__
* A common test used to determine the presence/concentration of a protein in a solution
* Enzyme-linked immunosorbent assay
* Enzyme-linked immunosorbent assay
33
New cards
* __*How does an ELISA work?*__
* Antibody recognizes and binds to the protein in solution,the attached enzyme causes a color change in a reagent
* Amount of color change depends on the number of antibodies that become bound to proteins in the sample
* Amount of color change depends on the number of antibodies that become bound to proteins in the sample
34
New cards
* __*What are monoclonal antibodies?*__
* Produced in cells through the fusing of immortal tumor cells with specific antibody producing cells(B cells)
* The resulting cells(hybridomas) grow and make large amounts of the specific antibodies that were coded for the original B-cells
* The resulting cells(hybridomas) grow and make large amounts of the specific antibodies that were coded for the original B-cells
35
New cards
What do biotechnlogists use protein abilities for?
* Biotechnologists use protein’s abilities to make huge amounts of novel/native proteins which are harvested from large volumes of cell cultures and used for many different things
36
New cards
* __*Where are the instructions for protein building kept?*__
* One or more structural genes on a DNA molecule of a chromosome
37
New cards
* __*How are gene expression and protein synthesis related?*__
1. Structural gene is rewritten in the form of mRNA(depending on the protein multiple mRNA transcripts may be created)
2. The mRNA transcript may be altered by having introns removed or may stay the same if functional
3. The functional mRNA strand floats to the ribosome where the nucleotide code is translated and a polypeptide strand of amino acids are complied
4. The polypeptide chain then folds into a protein due to the R-groups
5. Protein then can leave the cell or stay inside the cell
38
New cards
__***Transcription***__
genetic code must be rewritten onto a messenger molecule(mRNA)
39
New cards
* __*What happens during transcription?*__
1. Sections of DNA(genes) are unwound
2. The transcription enzyme, RNA polymerase, attaches to a promoter region at the beginning of a gene
3. The RNA polymerase reads the structural gene and builds a nucleic acid chain(mRNA) which is a complement to the strand being transcribed
40
New cards
* __*What does the transcription result in?*__
* mRNA molecule with a complementary code of a structural gene on the DNA strand
* Ex: If the DNA has a G or C or T or A then mRNA transcript will receive a C or G or A or U
* Ex: If the DNA has a G or C or T or A then mRNA transcript will receive a C or G or A or U
41
New cards
* __*Difference between RNA and DNA*__
* Ribose vs. deoxyribose
* Uracil vs. adenine
* Single stranded vs. double stranded
* Uracil vs. adenine
* Single stranded vs. double stranded
42
New cards
* Where does transcription occur in eukaryotic vs. prokaryotic cells?
* Eukaryotic: Nucleus
* Prokaryotic: DNA floating in the cytoplasm
* Prokaryotic: DNA floating in the cytoplasm
43
New cards
__***Translation***__
* the mRNA nucleotide code is rendered into a sequence of amino acids
44
New cards
* __*What is the process of translation?*__
1. mRNA strand(AUG,the start codon) attaches to the bottom subunit of a ribosome
2. Transfer RNA(tRNA) molecules pick up amino acids in the cytoplasm and shuttle them into the ribosome
3. The tRNA molecule brings the correct amino acids into place and the peptidyl transferase enzyme bonds the amino acids together with a peptide bond
4. The ribosome shifts to the next triplet codon and allows the next tRNA amino acid complex to attach
5. The mRNA codon chart will allow one to see the amino acid that will be added to the polypeptide strand for a given codon on the mRNA strand
6. Once the amino acid is added to the growing polypeptide chain the ribosome shifts one codon down the mRNA strand
7. Then another amino acid is added and so on until the ribosome reaches the stop codon(UAA,UAG,UGA)
8. Then the polypeptide chain disconnects
45
New cards
* __*What happens after translation?*__
* The polypeptide folds into a final three-dimensional configuration
* Some proteins may need modifications such as adding certain sugar groups(**glycosylation**), adding phosphate groups(**phosphorylation**), and/or splitting the polypeptide into one or more strands(**cleavage**)
* Once the folding and modification is done correctly so it is functional the polypeptide is considered a protein
* Some proteins may need modifications such as adding certain sugar groups(**glycosylation**), adding phosphate groups(**phosphorylation**), and/or splitting the polypeptide into one or more strands(**cleavage**)
* Once the folding and modification is done correctly so it is functional the polypeptide is considered a protein
46
New cards
* __*Name one very important group of proteins.*__
* Enzymes can **control** most steps in the production/breakdown of most biotech products
47
New cards
* __*How are enzymes used?*__
* Enzymes are added to reactions in test tubes to mimic reactions that naturally occur in cells
48
New cards
* __*Give one example of enzymes.*__
* Taq polymerase which is used for polymerase chain reaction
* During PCR a fragment of DNA is copied and recopied to produce million of identical fragments
* The enzyme, Taq polymerase, assembles the new DNA strands during PCR
* Scientists use this enzyme to create millions of DNA pieces in test tubes for research purposes
* During PCR a fragment of DNA is copied and recopied to produce million of identical fragments
* The enzyme, Taq polymerase, assembles the new DNA strands during PCR
* Scientists use this enzyme to create millions of DNA pieces in test tubes for research purposes
49
New cards
* __*What are enzymes?*__
* Proteins that act as catalysts that speed up biochemical reactions(building up/breaking down other molecules)
50
New cards
Give another example of an enzyme.
* __*Example:*__ DNA polymerase
* **DNA polymearse** puts nucelotides together into a growing DNA strand **vs.** **DNase** is an enzyme that speeds the breakdown of DNA into short chains of nucleotides or all the way down to individual nucleotides
* **DNA polymearse** puts nucelotides together into a growing DNA strand **vs.** **DNase** is an enzyme that speeds the breakdown of DNA into short chains of nucleotides or all the way down to individual nucleotides
51
New cards
* __*What must technicians learn with enzymes?*__
* Some enzymes are sensitive to environmental conditions and so technicians must learn how to handle these enzymes to ensure maximum enzyme activity
52
New cards
* __*Where do enzymes come from?*__
* In large amounts, they must be extracted from existing or engineered cells
* Manufactured enzyme products come from large fermentation tanks that ensure that concentrations and volumes are high enough to generate revenue
* Manufactured enzyme products come from large fermentation tanks that ensure that concentrations and volumes are high enough to generate revenue
53
New cards
* __*What are substrates?*__
* Molecules that enzymes act upon
54
New cards
* __*What is a characteristic of enzymes?*__
* Majority of enzymes are highly specific in that each enzyme only catilizes one type of chemical reaction and has one or only a few substrates
* **Example:** Proteases: break down large protein molecules into smaller molecules
* **Example:** Proteases: break down large protein molecules into smaller molecules
55
New cards
* __*What are biosynthetic enzymes?*__
* Enzymes that produce the molecules needed in organisms for structural purposes or other chemical reactions
56
New cards
* __*What are enzymes named after?*__
* Named after their substrates or the function they perform with “-ase” suffix
57
New cards
* __*What additional units do enzymes need to operate?*__
* Cofactors(ion,calcium,or a large organic compound known as a coenzyme)
58
New cards
__*Coenzymes*__
* Often a vitamin or part of a vitamin
* Ex: modified thiamin and niacin
* Ex: modified thiamin and niacin
59
New cards
__*Ions*__
* Ex: Ca^2+(Amylase needs this to break down start into sugar) or Mg^2+(DNA polymerase needs this)
60
New cards
When are enzyme genes expressed?
* __*Enzyme genes are expressed when mRNA is transcribed off the enzyme gene and is made at the ribosome*__
61
New cards
* __*What are the six categories of enzymes based on function?*__
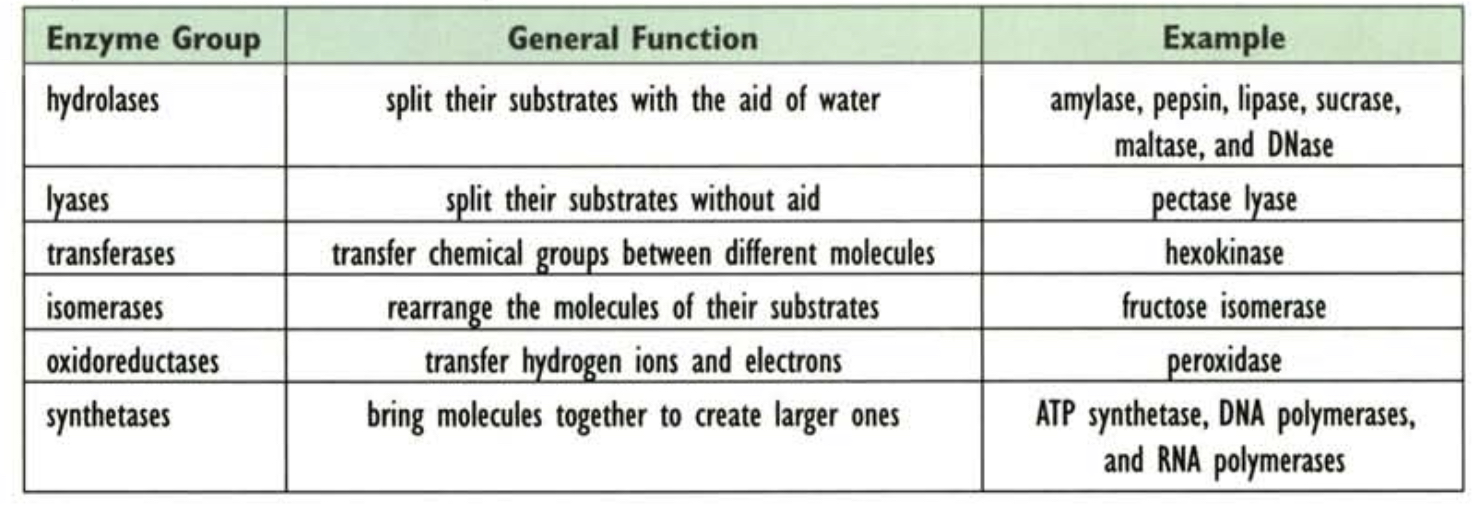
62
New cards
* __*When do enzymes begin to function?*__
* When enzyme and substrate become bound together at the active site
63
New cards
* __*What theories are out there that describe enzyme catalysis?*__
1. lock and key model: enzyme and substrate make an exact molecular fit at the active site which triggers the enzyme
2. induced fit model: substrate squeezes into an active site which induces enzyme activity
64
New cards
* What factors can affect enzyme activity?
1. Temperature: at higher temperatures to much stress is placed on hydrogen bonds causing enzymes to unravel
2. pH: The charged ions in high or low pH may interfere with an enzyme’s activity and cause it to denature
3. Salt concentration
65
New cards
* __*What is the optimum temperature?*__
* Temperature where enzyme is acting on a maximum number of substrate molecules
66
New cards
* __*What is Denaturation?*__
* Process of these enzymes losing their structures
67
New cards
* __*What is optimum pH?*__
* The pH at which an enzyme is most active
* How are enzymes maintained at their desired pH?
* Kept in buffers
* How are enzymes maintained at their desired pH?
* Kept in buffers
68
New cards
* __*How do scientists study proteins?*__
* They separate proteins from other molecules and determine their specific characteristics
69
New cards
* __*What characteristics do the majority of proteins have in common?*__
* Complicated 3-dimensional structure
* Polypeptide chain that makes up a protein is composed of 20 different amino acids in some length/order
* Number and arrangement of amino acids in the polypeptide determines the specific folding patterns
* Proteins have an overall net charge on the whole molecule(+ or -)
* Polypeptide chain that makes up a protein is composed of 20 different amino acids in some length/order
* Number and arrangement of amino acids in the polypeptide determines the specific folding patterns
* Proteins have an overall net charge on the whole molecule(+ or -)
70
New cards
* __*What techniques are used to study protein characteristics?*__
* Amino acid sequencing, mass spectrometry, and column chromatography
71
New cards
* __*How are proteins and small DNA molecules separated?*__
* **PAGE**(polyacrylamide gel electrophoresis)
* Have high concentration of molecules ranging from 4-18 %
* Have high concentration of molecules ranging from 4-18 %
72
New cards
* __*How is PAGE prepared?*__
* Made from scratch and poured in the lab or can be commercially prepared
* Recently, pre-poured gels are become more routinely purchased as the alternatives take a long time to prepare and are highly toxic
* Can be bought in 4-20 percent concentrations
* Recently, pre-poured gels are become more routinely purchased as the alternatives take a long time to prepare and are highly toxic
* Can be bought in 4-20 percent concentrations
73
New cards
**Gels with polyacrylamide gradients**
more concentrated on the bottom of the gel than on the top
74
New cards
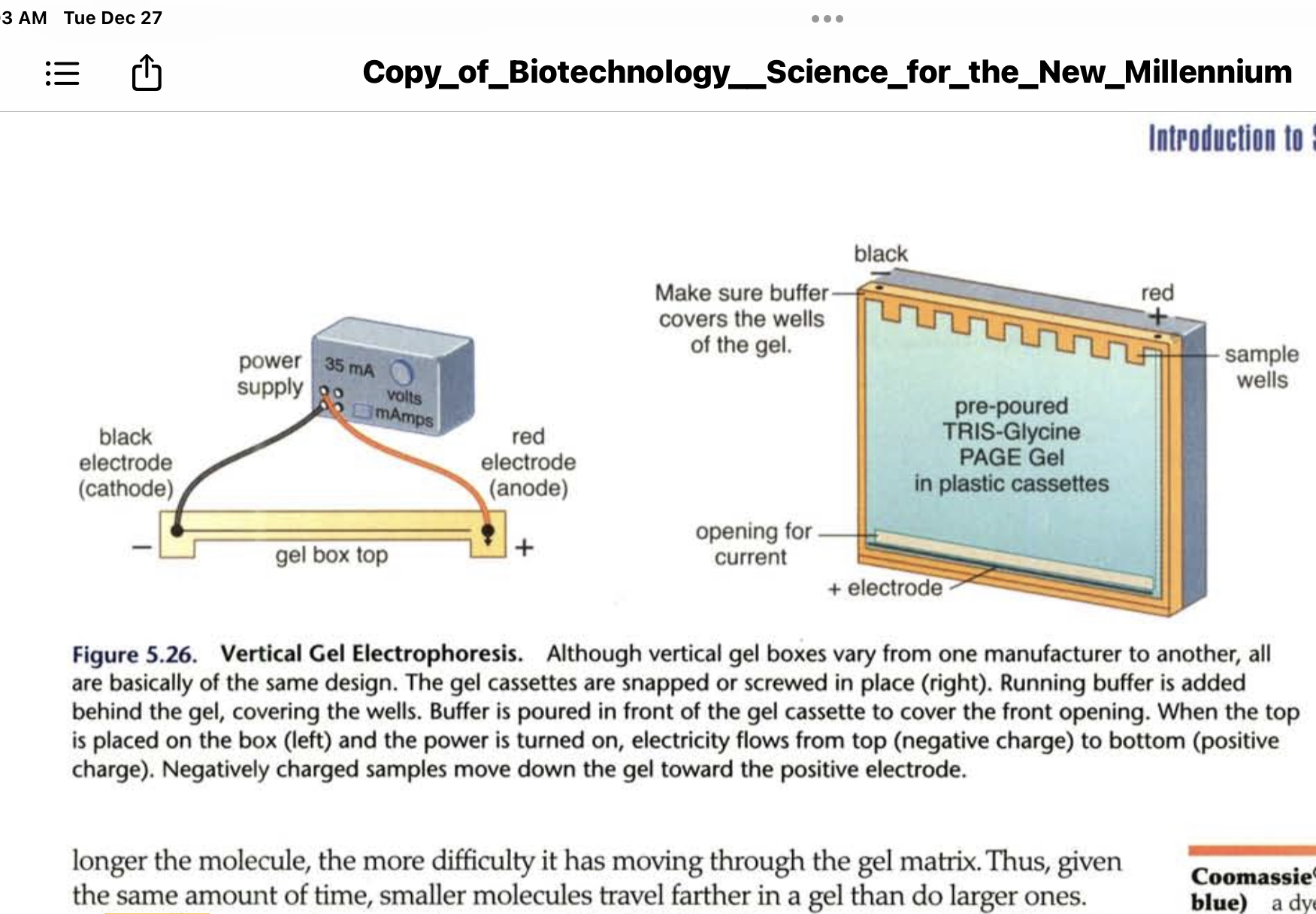
* **Vertical gels**
* Run in vertical gel boxes
* Have electrodes at opposite ends of the box
* When the correct buffer is placed in the box and a power supply is attached an electric field is created
* The samples in the vertical gel electrophoresis must have a net negative charge to be able to move into the gel and travel through it toward the positive side
* Smaller molecules travel farther in the gel than larger molecules do
* Have electrodes at opposite ends of the box
* When the correct buffer is placed in the box and a power supply is attached an electric field is created
* The samples in the vertical gel electrophoresis must have a net negative charge to be able to move into the gel and travel through it toward the positive side
* Smaller molecules travel farther in the gel than larger molecules do
75
New cards
* __*What are samples most commonly prepared for?*__
* PAGE with a special sample buffer containing a denaturing agent such as SDS(sodium dodecyl sulfate)
76
New cards
* __*What does SDS do?*__
* Dentures the protein to polypeptide chains so that each protein size is based on number of amino acid if contains, not on its shape
* Coats the polypeptide with a - charge so that all proteins have the same charge
* This allows polypeptides to move to the positive electrode only based on their size
* Present in loading dye(allows one to clearly see proteins)/buffer/gels along with additional **denaturing agents**(proteins are unwound to their primary structure when run)
* Coats the polypeptide with a - charge so that all proteins have the same charge
* This allows polypeptides to move to the positive electrode only based on their size
* Present in loading dye(allows one to clearly see proteins)/buffer/gels along with additional **denaturing agents**(proteins are unwound to their primary structure when run)
77
New cards
* What happens when samples are loaded into the wells and power is on?
* Polypeptides travel to the positive electrode at a rate proportional to their molecular weight thus showing the smaller the molecule the faster the move through the gel
78
New cards
* __*What do gels usually run at?*__
* 35 mAmp and can run anywhere from 1-3 hours
79
New cards
* What are the most popular stains?
1. Coomassie Blue: milligrams, expensive, requires much more time and labor, less sensitive
Used more often
2. Silver Stain: micrograms, more sensitive
80
New cards
* What do technicians observe after staining?
* Observes the protein-banding pattern for each sample to see how many peptide chains/proteins are present in a sample and the differences in protein sizes
* Molecular weight of the unknown bands can be determined by comparison
* Molecular weight of the unknown bands can be determined by comparison
81
New cards
* __*Protein Profile of Cells and Tissues*__
* Allows one to identify and quantify all of proteins present in a sample
* Comparing protein profiles can help one understand the differences in the structure or function of the tissue or cells allowing one to understand cell structure related diseases such as sickle cell disease
* Comparing protein profiles can help one understand the differences in the structure or function of the tissue or cells allowing one to understand cell structure related diseases such as sickle cell disease
82
New cards
* __*Protein Structure Affects Protein Function*__
* Ex: To understand how a muscle contracts and releases one must understand the structure of myosin
* Once one has understood this and gathered information from protein studies one can make realistic models of the protein’s structure which allows one to better understand the function of myosin in the muscle cell
* Once one has understood this and gathered information from protein studies one can make realistic models of the protein’s structure which allows one to better understand the function of myosin in the muscle cell
83
New cards
* __*Why do many scientists study proteins?*__
* Understand the chemical processes in cells
* How can this help the medical field?
* Example: Certain proteins trigger angiogenesis in tumors and certain tumors are known to produce proteins that encourage angiogenesis
* By learning to block these proteins tumors can be starved of their blood supply and die
* How can this help the medical field?
* Example: Certain proteins trigger angiogenesis in tumors and certain tumors are known to produce proteins that encourage angiogenesis
* By learning to block these proteins tumors can be starved of their blood supply and die
84
New cards
* __*What do you need to know to understand a protein’s function and mode of action?*__
* **Amino acid sequence** through protein sequencing
* **3 Dimensional structure** through x-ray crystallography and computer images
* **Charge**
* **Size** through PAGE
* **3 Dimensional structure** through x-ray crystallography and computer images
* **Charge**
* **Size** through PAGE
85
New cards
* __*What is often the cause of the majority of genetic disorders?*__
* The lack of activity of various proteins
**Example**: abnormal cell membrane transport protein causes cystic fibrosis
**Example**: abnormal cell membrane transport protein causes cystic fibrosis
86
New cards
* __*Why are protein studies often conducted?*__
* Understand evolution and taxonomic relationships
* **Example**: Amino acids in human hemoglobin molecules is 98% the same as in chimpanzee hemoglobin while a horse has 78%
* This illustrates the closer ancestry between humans and chimps than between horses and human
* **Example**: Amino acids in human hemoglobin molecules is 98% the same as in chimpanzee hemoglobin while a horse has 78%
* This illustrates the closer ancestry between humans and chimps than between horses and human
87
New cards
* __*Differences in Proteins*__
* By comparing proteins one can see the differences in the proteins which hints at differences in DNA
* This leads to the conclusion that changes in DNA from one species to another indicate evolutionary change
* This leads to the conclusion that changes in DNA from one species to another indicate evolutionary change
88
New cards
* __*Similarities in Proteins*__
* The degree of similarity in proteins allows scientists to make inferences about evolutionary history of different species
89
New cards
* __*What must be done before biotech products are comemercially produced?*__
* Many studies have to be conducted to how to properly store/use these proteins
* Chemical behavior of protein in different environments is critical for designing different protocols
* Chemical behavior of protein in different environments is critical for designing different protocols
90
New cards
* __*What can happen once a protein’s molecular weight,charge,or shape is found out?*__
* It can be isolated through colum chromatography that are confined by visualizing purification fractions on gels
91
New cards
* __*What staff typically make up a huge amount of scientific staff at universities,government agencies, and biotech companies?*__
* Protein researchers/technicians
92
New cards
* __*Who funds basic protein research done by these staff?*__
* Shareholders or grants from government agencies and nonprofit foundations( American Cancer Society)
93
New cards
* __*What has been recently discovered by scientists that could be sources of biotech products?*__
* Plant and animal parts
* Example: the pancreas of many kinds of livestock is ground up and used as a source of the protein insulin
* Example: the pancreas of many kinds of livestock is ground up and used as a source of the protein insulin
94
New cards
When is it impractical to extract a potential product from a natural source?
* from a natural source?
* When it is made in small quantities in nature
* **Example**: t-PA(tissue plasminogen activator) is made in small quantities and can’t be harvested from the blood
* This is why scientists took Chinese hamster ovary cells(CHO) which took up human DNA and transcribe it into the t-PA protein
* These cloned cells were kept in large fermentation tanks in a large quantities in a broth culture in which the t-PA is harvested and purified from
* **Example**(2): Production of large quantities of Antibiotics(molecules produced in bacteria and fungi to inhibit the growth of other bacteria)
* In conclusion, some biotech products are harvested directly from nature, synthesized in a lab, while others are cloned genetically engineered organims
* When it is made in small quantities in nature
* **Example**: t-PA(tissue plasminogen activator) is made in small quantities and can’t be harvested from the blood
* This is why scientists took Chinese hamster ovary cells(CHO) which took up human DNA and transcribe it into the t-PA protein
* These cloned cells were kept in large fermentation tanks in a large quantities in a broth culture in which the t-PA is harvested and purified from
* **Example**(2): Production of large quantities of Antibiotics(molecules produced in bacteria and fungi to inhibit the growth of other bacteria)
* In conclusion, some biotech products are harvested directly from nature, synthesized in a lab, while others are cloned genetically engineered organims
95
New cards
* __*What is Amylase?*__
* Enzyme that is produced by several organisms and is made to break down the polysaccharide amylose(plant starch) to maltose
* Maltose is in turn degraded to the monosaccharide glucose by maltase
* Maltose is in turn degraded to the monosaccharide glucose by maltase
96
New cards
__***Amylase Market Size***__
* Amylase has a large industrial market due many industries needing its properties of being able to easily and efficiently breaking down starch/produce sugar
* **Ex**: Textile industry needs amylase to quickly remove starch from fabric
* More specifically many industries such as the beverage industry can use Amylase to breakdown starch to sugar and this is the more economical alternative in comparison to an island-grown sugar
* **Ex**: Textile industry needs amylase to quickly remove starch from fabric
* More specifically many industries such as the beverage industry can use Amylase to breakdown starch to sugar and this is the more economical alternative in comparison to an island-grown sugar
97
New cards
__***Amylase Product Sources***__
* __Humans__ produce amylase in salivary(saliva) glands and in their pancreas
* __Decomposing bacteria/fungi__ also use this enzyme to break down plant molecules for food
* Ex: Bacillus Subtilis(can be used to create active amylase if scientists are able to grow it)
* Scientists at a biotech company made rAmylase by developing a production process that involved using genetically engineered E.coli(easy to use) bacteria to produce the recombinant alpha amylase on a large scale
* __Decomposing bacteria/fungi__ also use this enzyme to break down plant molecules for food
* Ex: Bacillus Subtilis(can be used to create active amylase if scientists are able to grow it)
* Scientists at a biotech company made rAmylase by developing a production process that involved using genetically engineered E.coli(easy to use) bacteria to produce the recombinant alpha amylase on a large scale
98
New cards
* __*What is amylase function in the body?*__
* Amylase is secreted onto ingested food allowing the starch in the food to be digested as absorbable sugar which body cells can use as fuel
99
New cards
* __***Amylase Comprehensive Product Development Plan***__

100
New cards
* __*What is meant by the term assay?*__
* Synonymous with the word test