UTA CHEM 1442 Rogers Exam 2 (Ch.13 & 14)
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
Chemical Kinetics
The rates or speeds at which chemical reaction occurs.
What influences the rate of reactions?
1. Temperature
2. Reactant concentration (pressure)
3. Presence or absence of a catalyst.
4. Nature of reactions
i. aq reaction = fast
ii. Large molecules w/ strong bonds tend to be slow.
5. In a heterogeneous reaction the state of sub-division of the condensed phase.
Catalyst
substance that speeds up the rate of a chemical reaction without being consumed
Rate Constant; k
dependent only on temperature and presence or absence of a catalyst.
the bigger the k the faster the reaction.
Rate law must be determined
experimentally
The exponents in the rate law are referred to as the
order of the reaction
The sum of the exponents is called the
overall order of the reaction
rate=k[A][B]
A: 1st order
B: 1st order
Overall: 2nd order
rate=k[A]^2[B]
A: 2nd order
B: 1st order
Overall: 3rd order
first order reaction
rate=k[A]
second order reaction
Rate=k[A]^2
zero order reaction
rate=k
rate is constant, straight diagonal line when graphed
Rate constant
1. k varies w/ temperature; therefore temperature must be specified.
2. a reaction w/ a large k is FASTER than one w/ a smaller k.
3. k is independent of reactant concentration
integrated rate law first order
ln[A]t = -kt + ln[A]0
a plot of ln[A] vs. time gives a straight line with a slope of -k
![<p>ln[A]t = -kt + ln[A]0</p><p>a plot of ln[A] vs. time gives a straight line with a slope of -k</p>](https://knowt-user-attachments.s3.amazonaws.com/cf6a93e4-32f2-4ae8-92e6-a57486bff164.jpg)
The half-life of a first order reaction is
independent of the initial concentration
half-life equation of a first order reaction
t1/2 = ln2/k
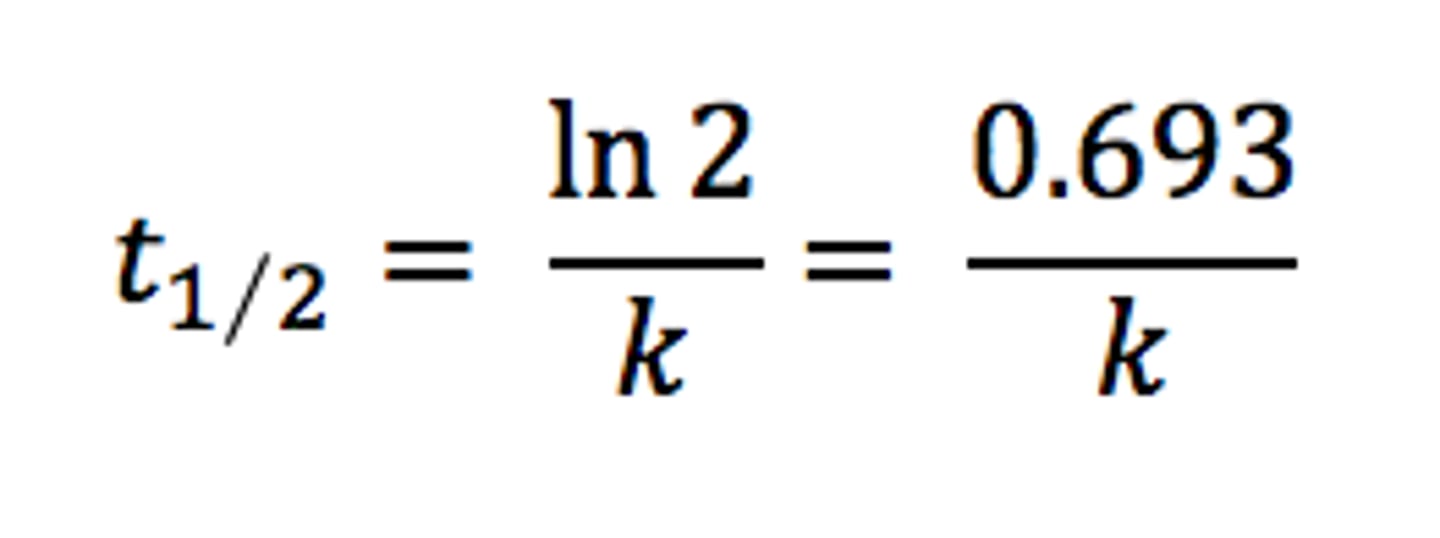
The most important reason for studying chemical kinetics is in order to determine
reaction mechanism
reaction mechanism is
a particular sequence of events leading to the overall chemical change
Molecularity
how many particles are involved in an elementary reaction
Unimolecular
an elementary reaction that involves a single molecule
HBr --> H + Br
rate=k[A]
Bimolecular
an elementary step in a reaction that involves two particles
H + Br --> HBr
rate=k[A][B] or rate=k[A]2
Termolecular
simultaneous collision of three molecules (rare)
H2 + Br + Cl --> HBr + HCl
rate=k[A][B][C] or rate=k[A]2[B]
You cannot get the rate law from the _________ but you can get the rate law from _________.
overall balanced equation; an elementary reaction or an elementary step.
Multi step mechanism must satisfy two requirements
1. it must agree w/ the experimentally determined rate law.
2. the sum of the individual elementary step must equal the overall balanced equation.
reactive intermediate
formed in one step and then consumed in a subsequent step. (NO3 in example below)
NO2 + NO2 --> NO3 + NO slow, rds
NO3 + CO --> NO2 + CO2 fast
________________________________________
NO2 + CO --> NO + CO2
two observations of the collision model
first, increasing the temperature of a reaction will increase the reaction rate.
second, increasing the temperature of a reaction will increase the magnitude of the rate constant k.
In order for a reaction to occur, molecules must
1. Collide
2. Collide w/ sufficient energy
3. Collide w/ appropriate orientation in space
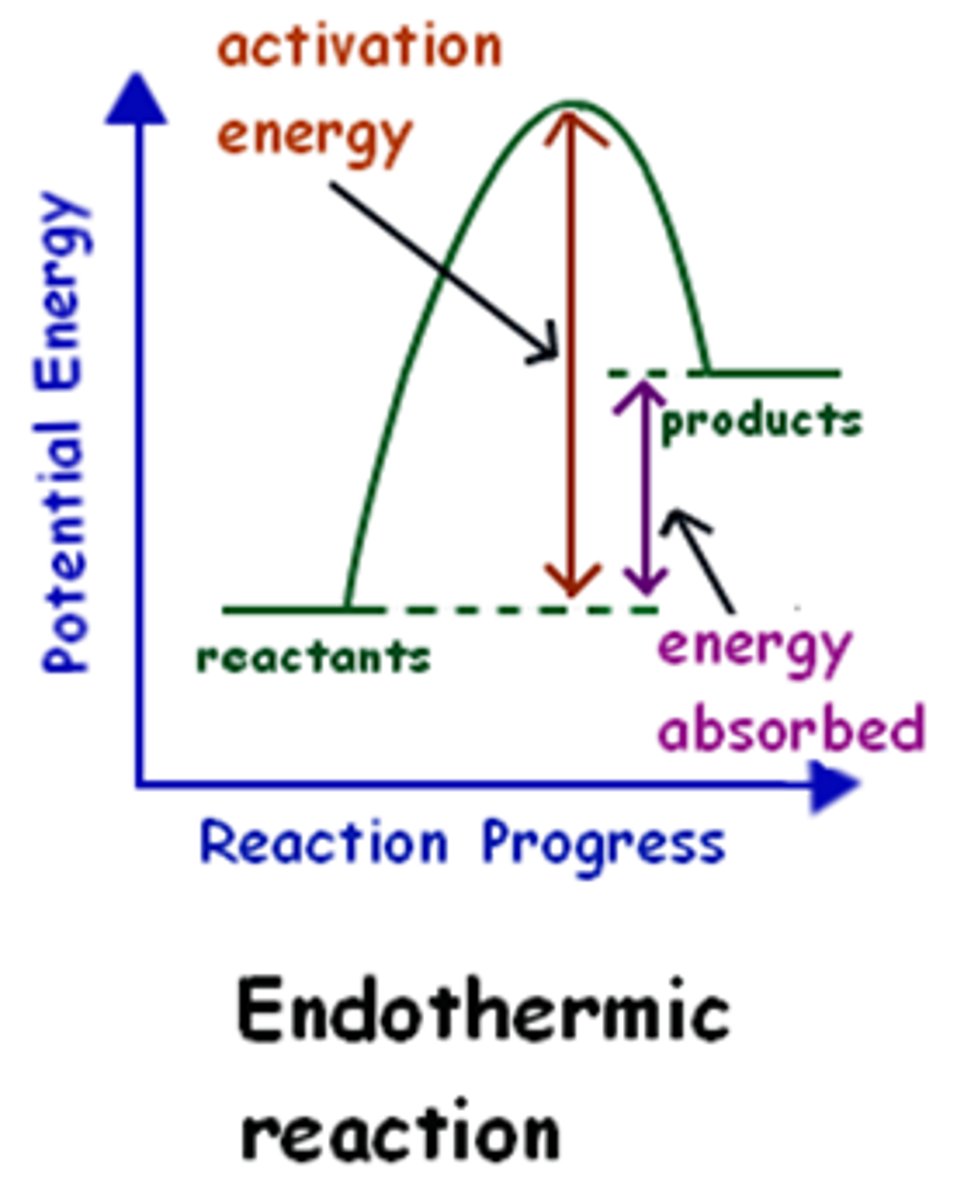
Endothermic reaction profile
products are higher than reactants
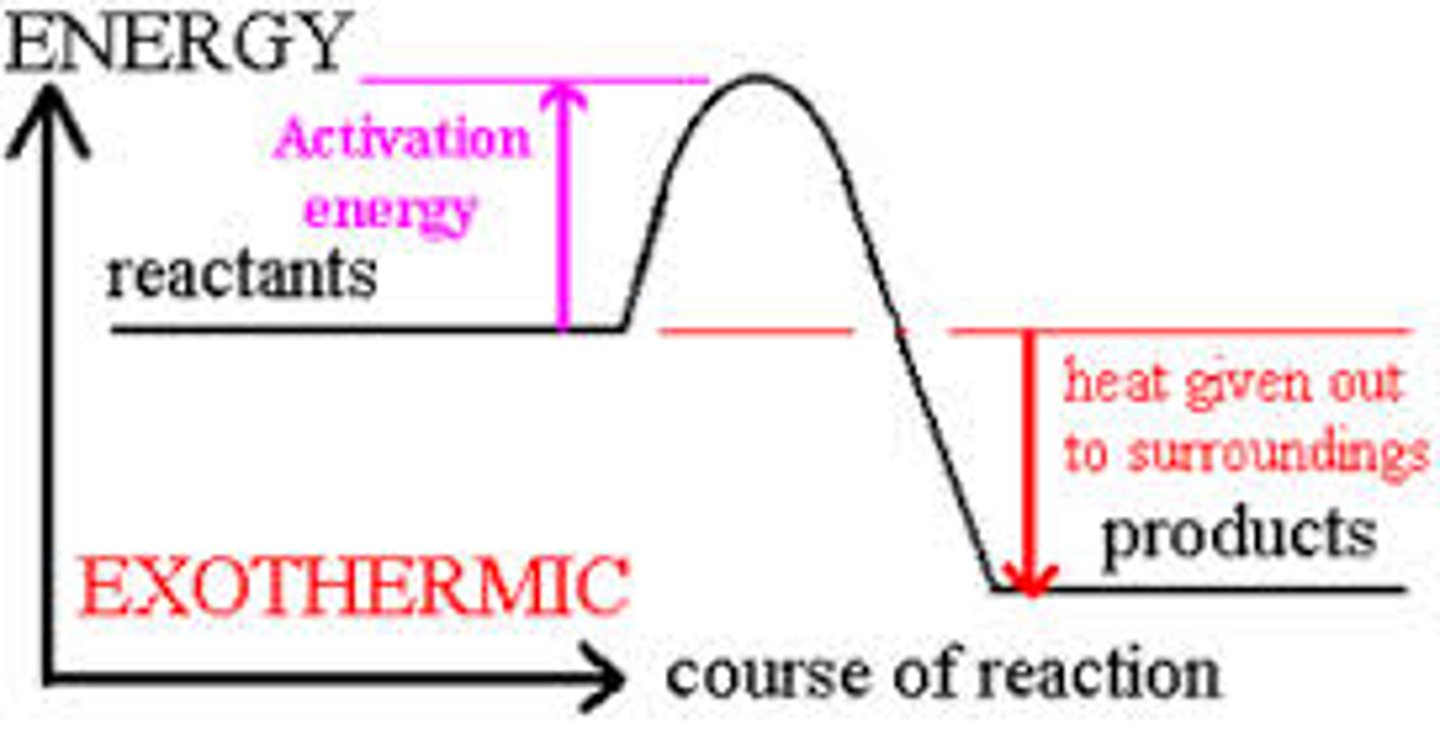
Exothermic reaction profile
reactants are higher than products
two important principles about Ea (activation energy)
1. The higher the Ea, the slower the reaction
2. A catalyst speeds up a reaction by lowering the Ea
How does a catalyst increase the rate of a reaction?
by lowering the Ea
Does increasing temperature change the Ea?
No
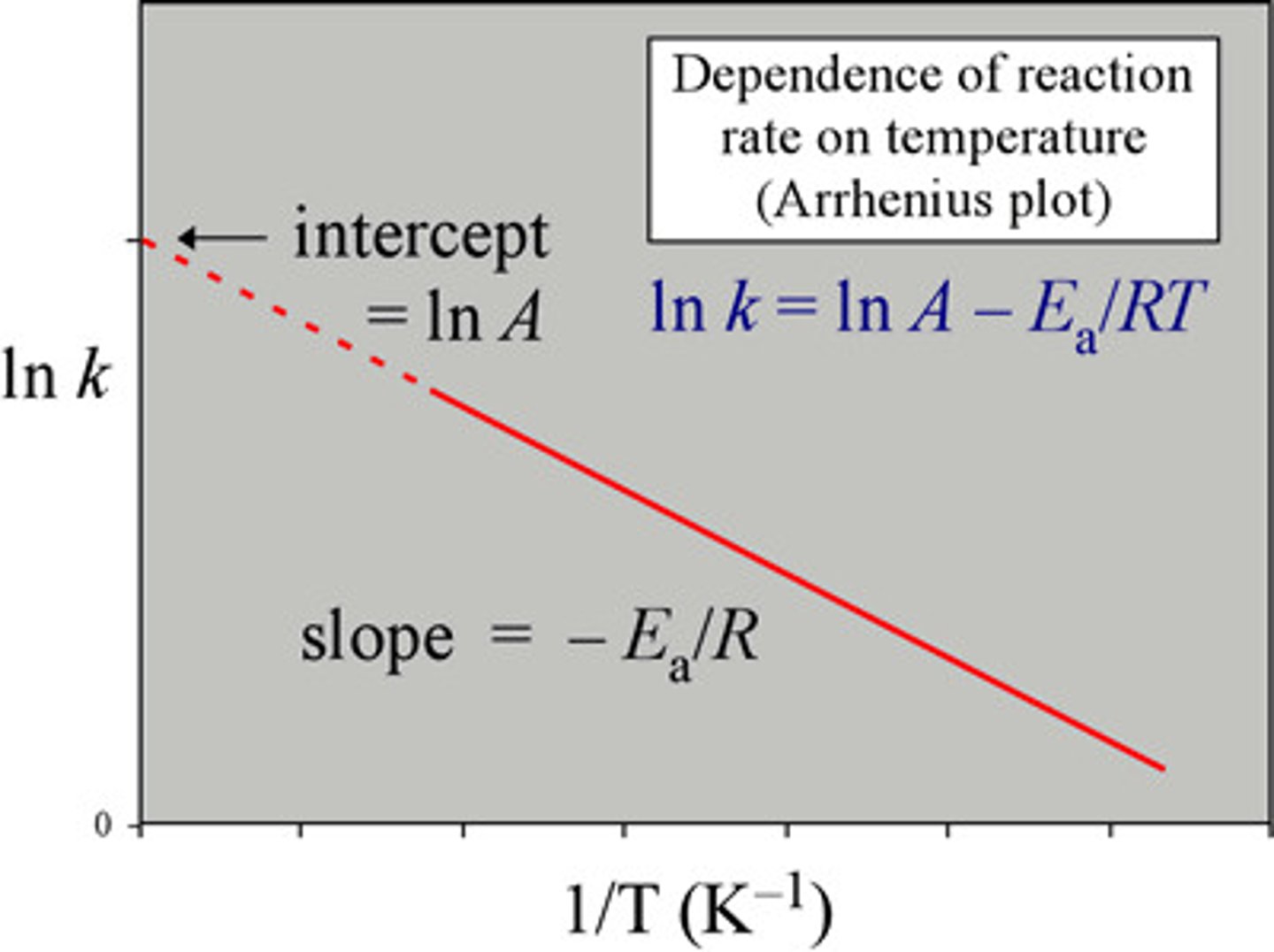
Arrhenius equation
k=Ae^(-Ea/RT)
k= rate constant at temperature (K)
A= pre-exponential term
e= base of natural log
Ea= activation energy (kJ/mol x K)
R= 8.314 J/mol x K = 8.314x10^-3 kJ/mol x K
Easier Arrhenius equation
lnk = -Ea/R(1/T)+lnA
Arrhenius plot
dynamic equilibrium
A state in which no net change occurs because there are two opposing processes occurring at the same rate.
Two characteristics of chemical equilibria
1. two opposing processes occur at the same rate
N2 + 3H2 --> 2NH3
2NH3 --> N2 + 3H2
2. at equilibrium the concentrations of reactants and products do not change with time.
Equilibrium constant for Kc
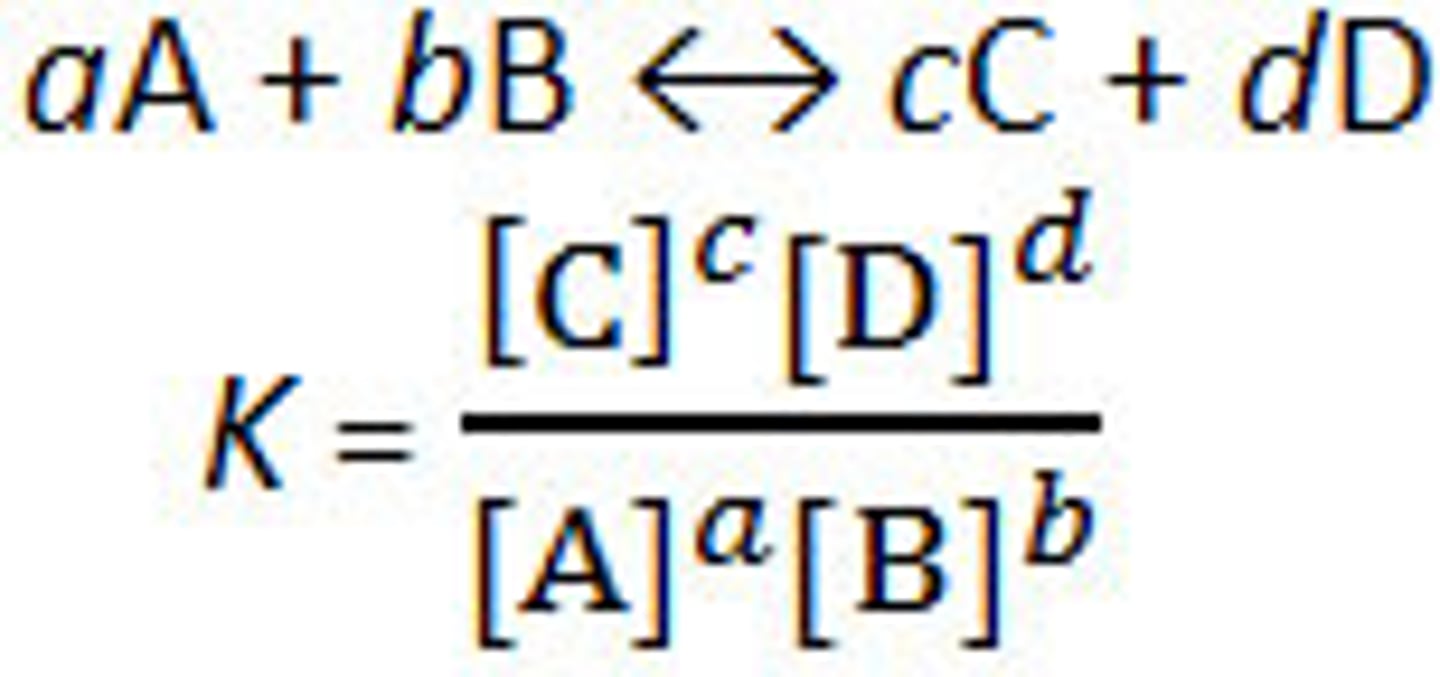
Equilibrium constant for Kp
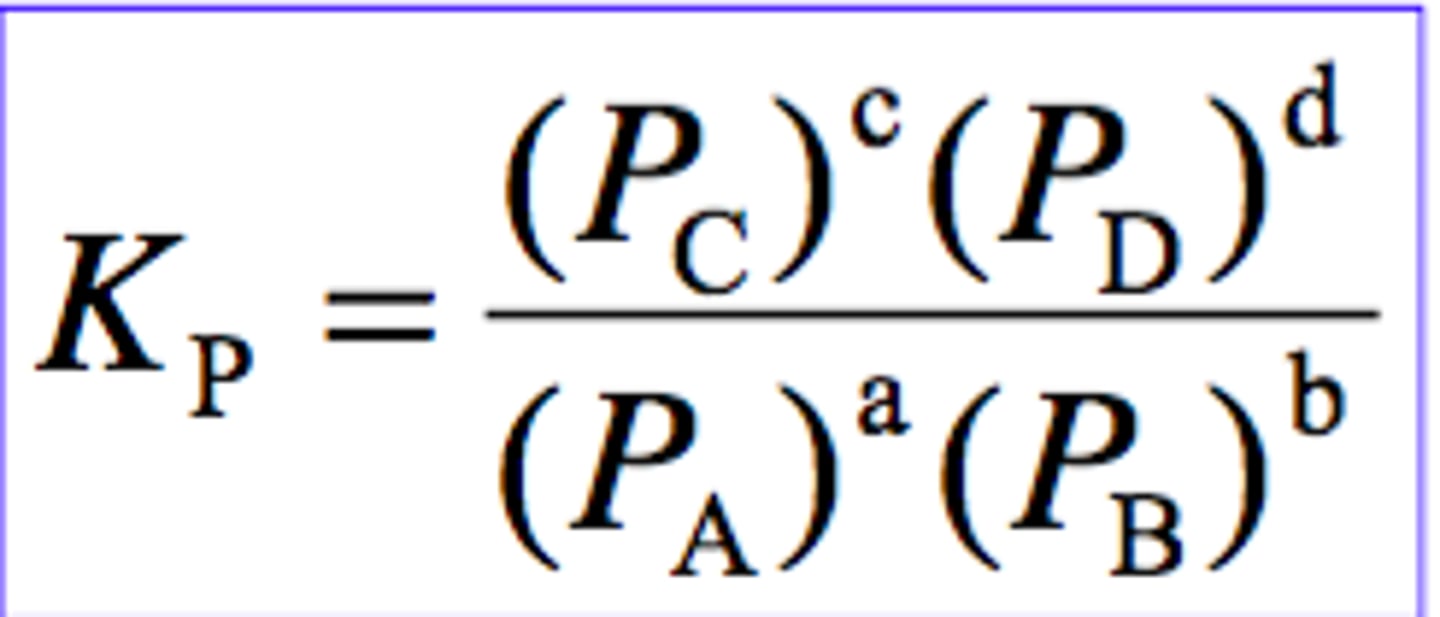
Keq > 10^3
mostly products at equilibrium
Keq < 10^-3
mostly reactants at equilibrium
Keq describes
the extent of the reaction
Reaction quotient, Qc
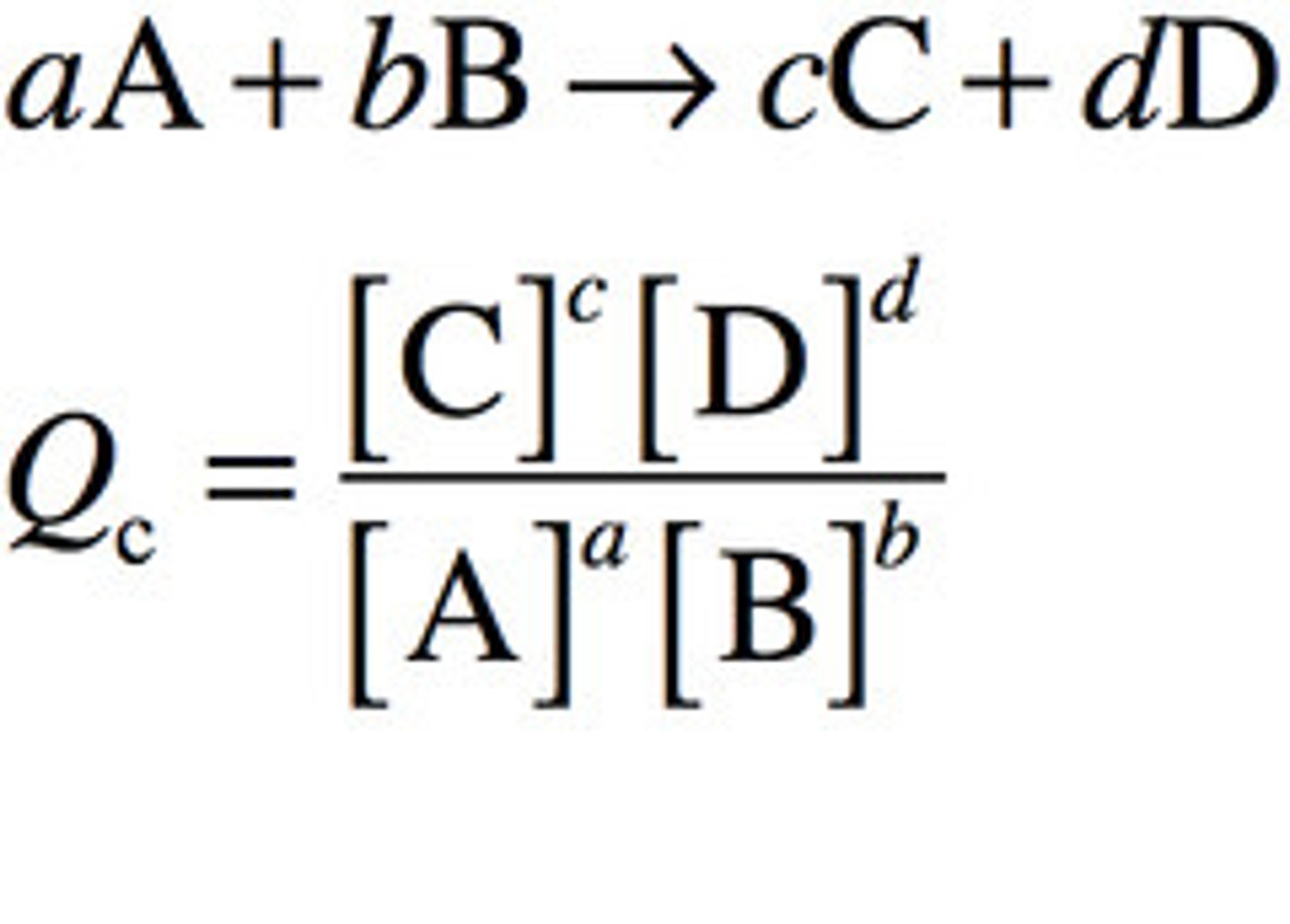
Qc = undefined
1. none when 0/1
2. reverse reaction occurs when 1/0
Qc < Kc
the forward reaction predominates
Qc = Kc
the system is at equilibrium
Qc > Kc
the reverse reaction predominates
if the equation is reversed:
A --> 2B
2B --> A
Kc= [B]2/[A]
Kc'= [A]/[B]2
Kc'= 1/Kc
Heterogeneous Equilibria
ALWAYS omits any solid or liquid for and Keq equation. Because concentration (M) of a solid or liquid is an intensive (doesn't depend on how much you have) property-- like density (mass/vol)
CaCO3(s) <---> CaO(s) + CO2(g)
Kc= [CO2]
Kp= Pco2
Equilibria occurring in aq solutions
Omit H2O(l) from Keq equation
Kp=Kc(RT)^Δn
Δn = the change in the number of moles of gas in the FORWARD reaction
R= 0.08206
Px= must be in atm