Chapter 6 - Rate Of Reactions
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
Rate Of A Reaction
Change in concentration of a product or reactant in a given time period
Can be change gas, pH, colour
given in mol L-1 s-1 (moles per litre per second - how much is being depleted)

Easier way fro rate of reaction
How much of products is being used over a period of time
How much of reactants is being used over time
Using Volume Of Gas
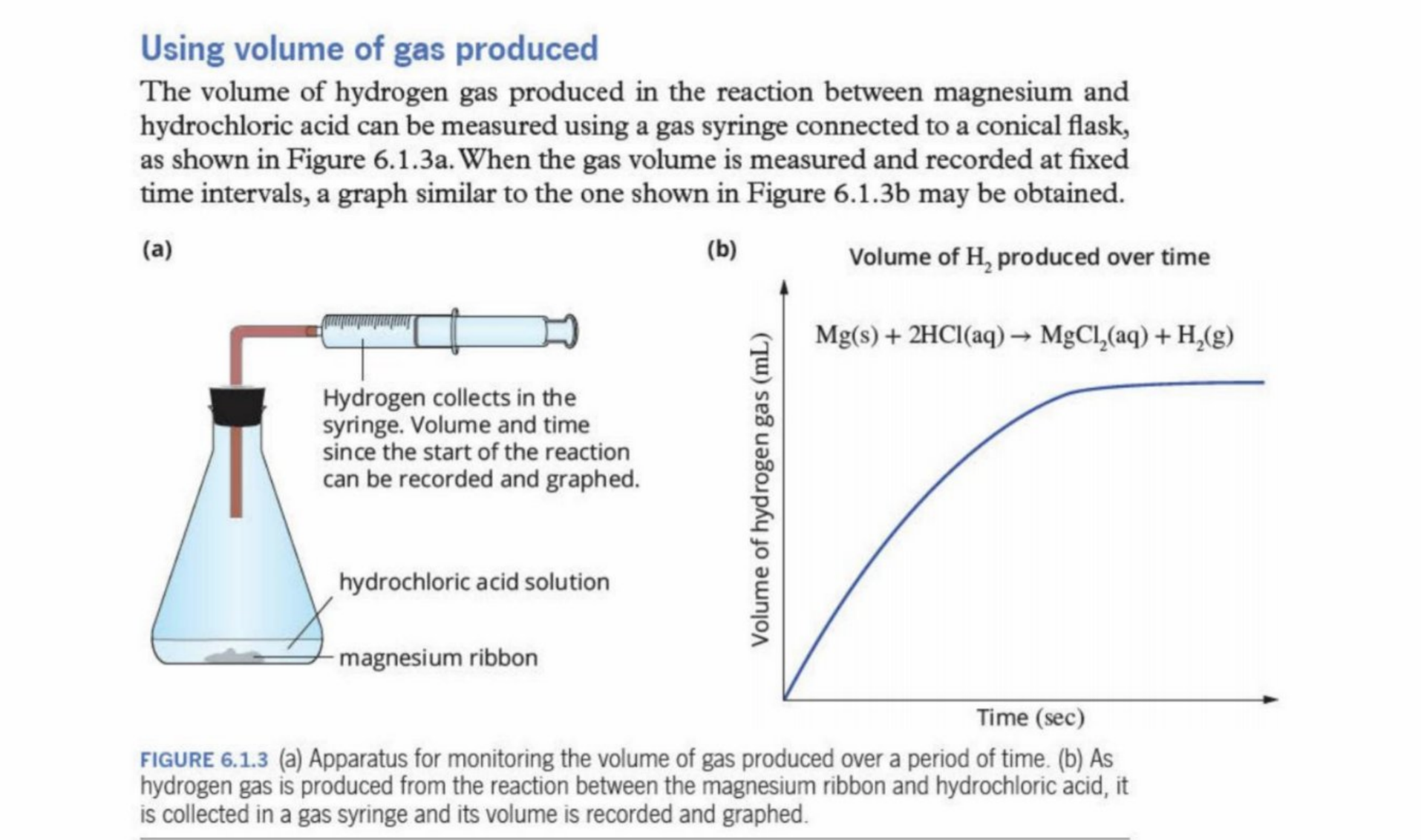
Explanation of Example
A steeper gradient indicates a higher rate of reaction
Eventually, it evens out - less reaction occurring
Becomes zero - reaction has concluded
Rate of reaction can be calculated through gradient of graph
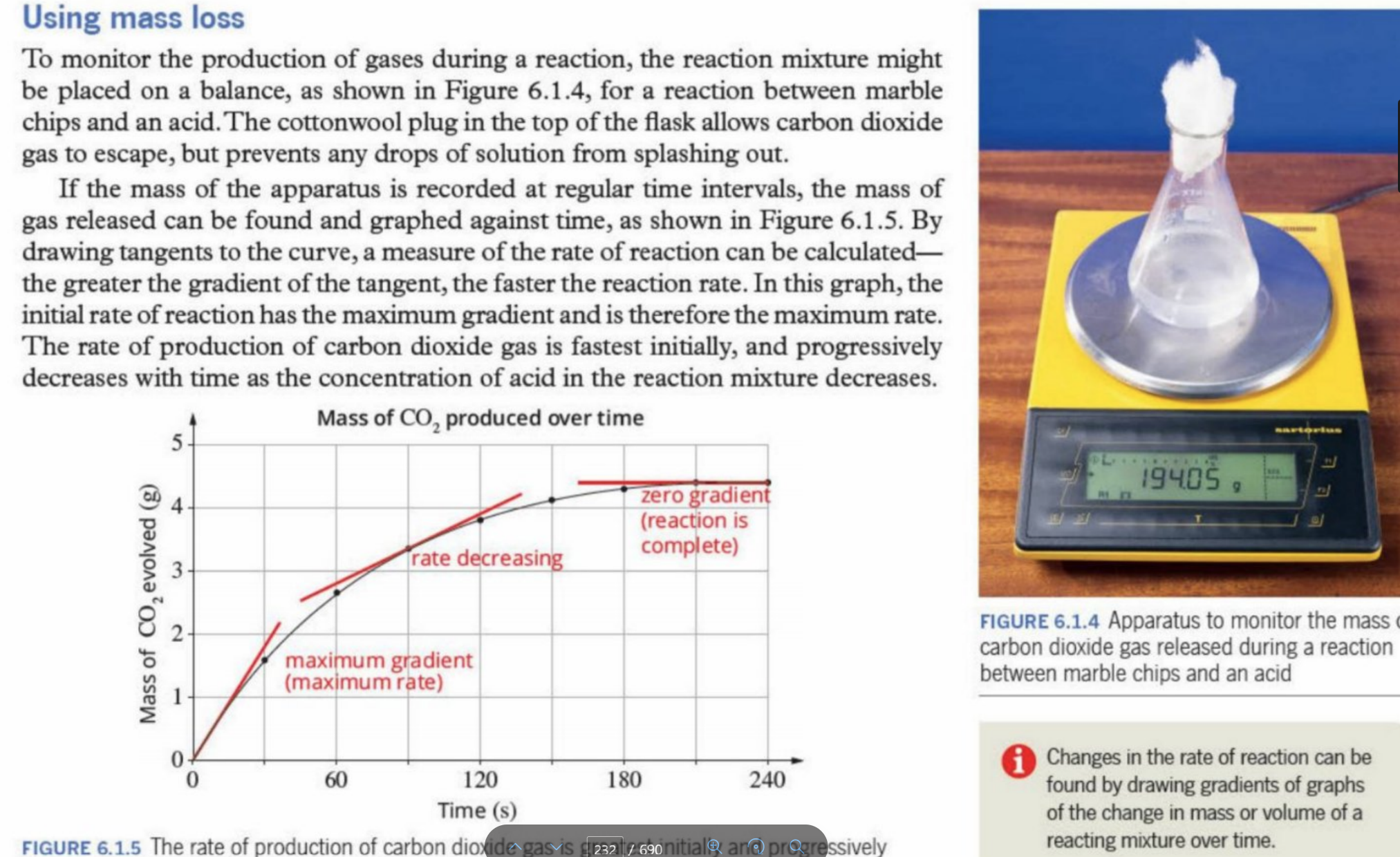
Using Mass Loss
By measuring weight of apparatus after during regular intervlas (CO2) gas is lost
Conditions For A Reaction To Take Place (Collision Theory)
Must collide with each other
Must collide with sufficient force to break bonds within reactants
Must collide in the correct orientation to break bonds among reactants and allow the formation to create new products
If these are not satisfied = no reaction
Meeting Ea threshold
Minimum amount of energy for a reaction to occur (energy developed through collisions)
Particles are always constantly colliding (typically collisions have less energy than Ea) - the kinetic energy turns into potential energy
External stimulus (light/heat/sound) - can increase particle motion, leading to a greater collision - surpassing Ea for a reaction
Exothermic Vs. Endothermic (Unclear)
Endothermic: Products are higher energy than reactants, more external stimulus required for an appropriate collision
Exothermic: Products have a lower energy than reactants, so only a small amount of external stimulus is required
Endothermic Vs. Exothermic
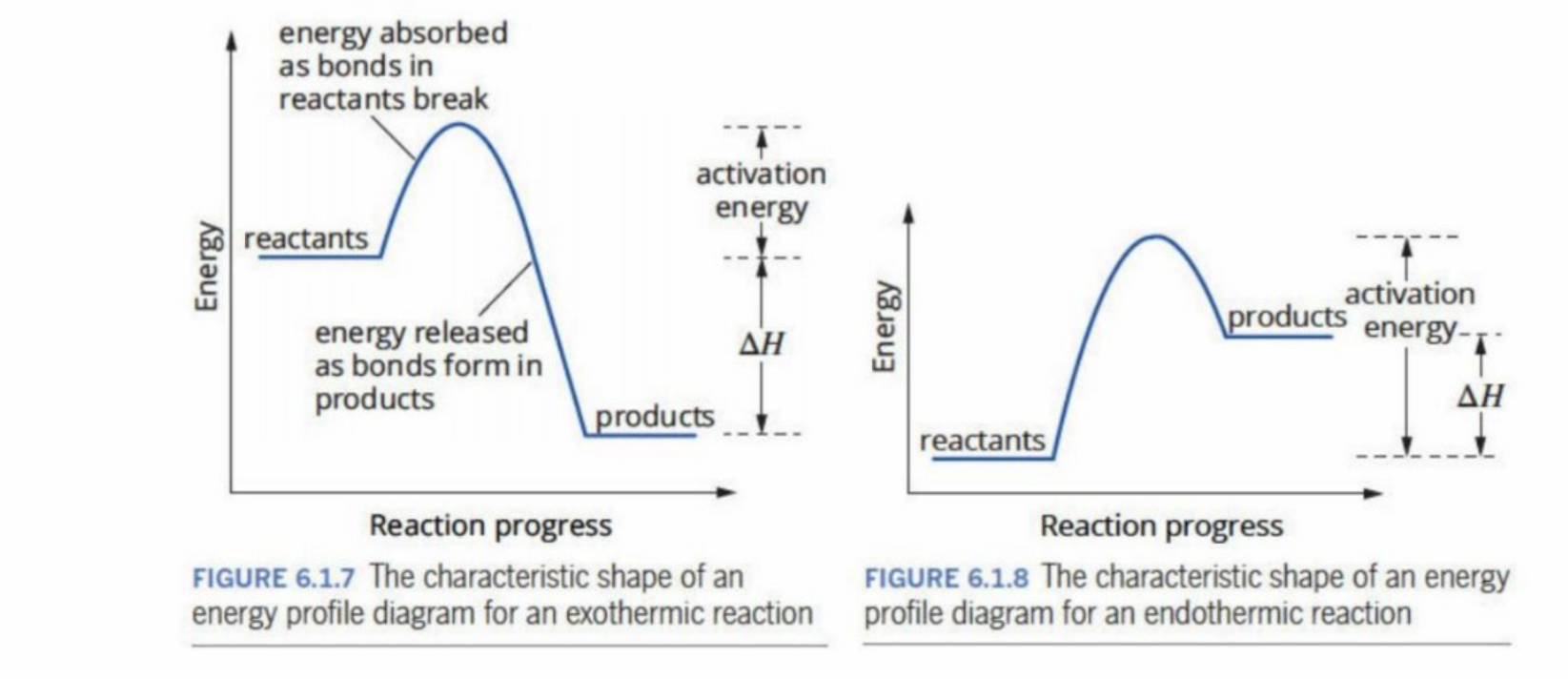
Transition State/Activated Complex
The site of maximum potential energy within a reaction
Bond-breaking (reactants), bond-forming (products) is being done
Is highly unstable
Like a “hill” - can go back to being products, or go back to being reactants (determined by relative energies of the products and reactants and random molecular motion) - the activated complex is most likely to move towards the side with less energy
e.g. in exothermic - products have less energy - favors the forward reaction
in endothermic - products have more energy - more likely to fall back to reactants, unless sufficient energy is provided
In reversible reactions, and equilibrium may be formed
Equilibrium
Forward reactions = Backward reactions
Forward Reactions - Transition state to products
Backward Reactions - Transition state to reactants
On average, no. of forward reactions = no. of backward reactions
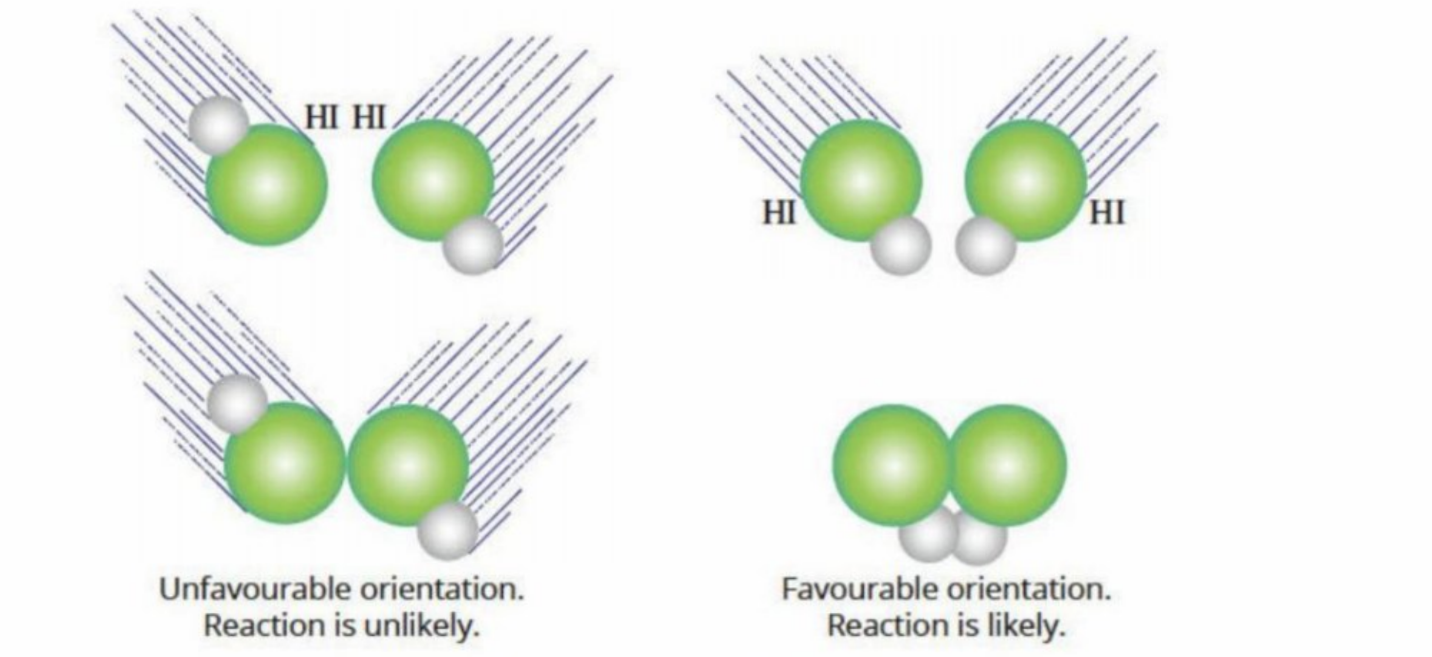
Orientation Of Colliding Molecules
The correct orientation of collision must occur - if not, no matter how great the activation energy, there will be no reaction
Must react in a specific orientation allowing the bonds in the reactants are broken and bonds in the products are formed (bonds must break entirely, not partially)
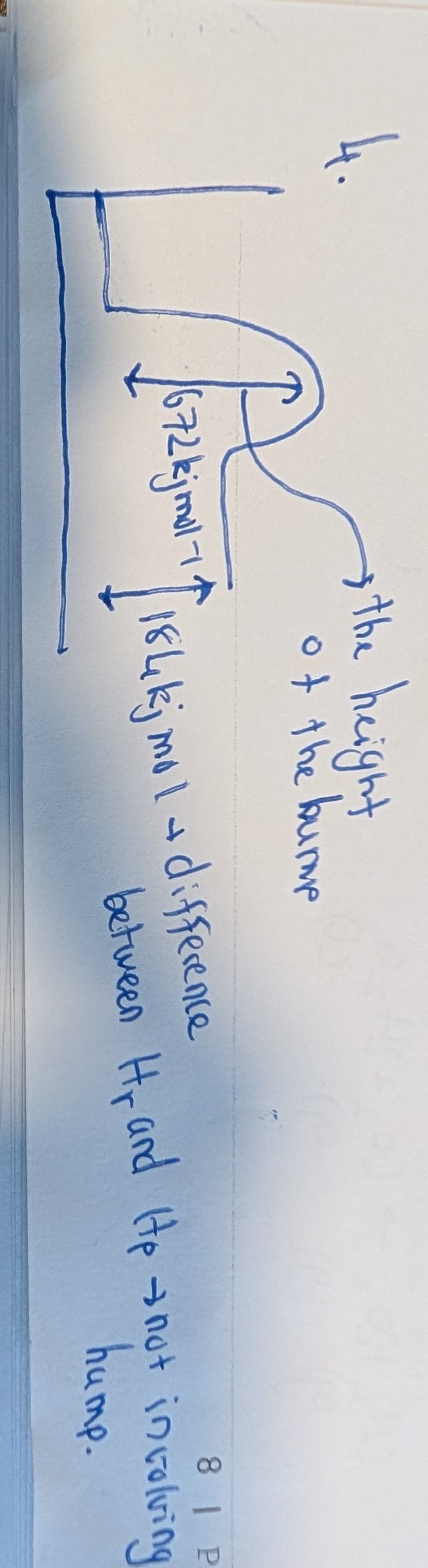
Finding Activation Energy Of Reverse Equation (and Enthalpy)
Ea of reverse = Ea of foward - Enthalpy
-(Enthalpy) = new Enthalpy of reverse equation
If a reaction is endothermic, its reverse is always exothermic (and vice versa)
How Rates Of Reactions Can Be Altered
Increasing surface area of solid reactant
Concentration of reactants in a solution (more collisions)
Pressure of gaseous reactants
Temperature of the reaction
Prescence of a catalyst
In order to increase reaction rate
Increase the frequency of successful collisions
Increasing the energy of all collisions so that the proportion of collisions that have energy higher than Ea is higher
Increasing concentration/Pressure
When concentration increases (more particles in a given area) then subsequently the frequency of collisions for which E (energy of collision) > Ea also increases
When adding more gas into a fixed volume or a smaller volume, it increases the concentration of gas molecules = more successful collisions
Increasing Surface Area
By expanding surface area (for a solid), more successful reactions can occur, as they only happen at the surface
More reactant particles are exposed, which results in more successful collisions, triggering a reaction
e.g. burning paper than a log - has more surface area so it catches fire, and burns easier

Higher oxygen concentration in shallow water causes more frequent successful collisions, increasing the rate of corrosion.
Common sense- oxygen drops when the water gets deeper
In shallow waters, water has a higher concentration to oxygen atoms and comes in contact with temperatures high than deep water, effectively increasing the rate of the reaction
Though more pressure is found in deep water, it is not enough to mitigate the higher concentration of oxygen atoms in shallow waters
Increasing Energy Of Particles
Only occurs through increase of temperature
Relation between kinetic energy and temperature
KE = 1/2mv2
When the temperature increases, v increases (v is the temp, m is the mass)
Since v is squared, a small increase can result in much more kinetic energy, resulting in more reactions
Greater proportion of collisions will have E> Ea - will have energy that is greater than of = to the required activation energy
Mxwell-Boltzmann Distribution Curve
Mean - the average of energies across all particles (is boosted by th particles in the high end)
Most probable energy point - The energy level that most particles will have
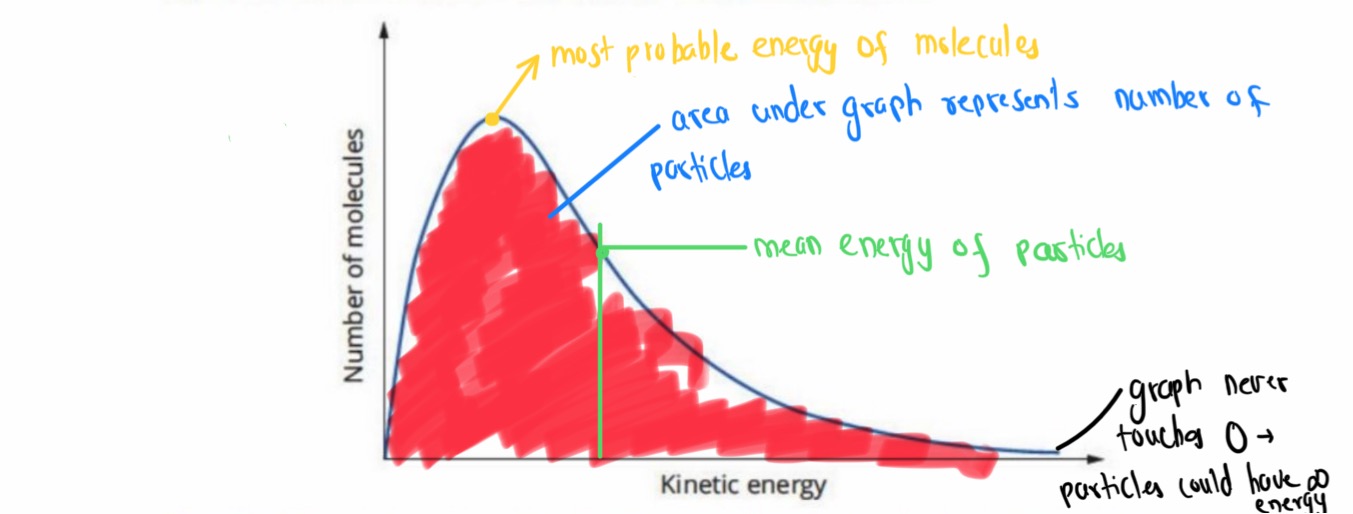
Increasing Temperature/ Adding Catalyst - Effect On Boltzmann Graph

When temperature increases, does activation energy decrease?
No - only proportion of particles that can overcome the Ea barrier increases - not the physical barrier itself
What happens if a catalyst itself has larger surface area
It provides more reaction sites between the particles and the catalyst - leads to accelerated reaction
Role Of Catalysts
Reduces required activation energy, and introduces an alternative pathway for the reaction to take place (less activation energy required), and lowers the energy level of the transition state
Normally reactants must get over a high activated complex, but through catalysts they can go through another pathway that reduces the height of “the hill” required
How Catalysts Lower Activation Energy
Bringing reactants together correctly
Increases the frequency of effective collisions
Ensures correct orientation for bond breaking/forming
🔹 Weakening existing bonds
The catalyst may temporarily bond with reactants
This weakens bonds, so less energy is needed to break them
🔹 Stabilising the transition state
The transition state becomes lower in energy
A lower-energy transition state = lower activation energy
Key Points To Catalystr Interaction
Activation energy decreases
Enthalpy remains the same (smaller bump in activation energy only)
Catalyst is not consumed (is still chemically unchanged, and is present in the mixture at the end)
Catalyst Effect

Types Of Catalysts
Homogenous Catalysts, Heterogenous Catalysts
Homogenous Catalysts
Are in the same form as the reactants (solid, liquid, gas)
Mixes uniformly with reactants
Creates temporary intermediates
Has good contact with reactants
Catalyst is regenerated in the end
Temporary Intermediates
When the catalyst makes bonds with the reactants for a very short time, and allow the reaction to occur in multiple smaller steps
Each step has lower activation energy - thus activation energy is lower
Are consumed before the reaction finishes (not in overall reactions)
Another explanation: A short-lived species formed during a reaction that exists between reactants and products, but is not the transition state. -Has lower energy than the transition state
Heterogenous Catalysts
Catalysts that have a different form to the reactants (Usually a solid when reactants are gases and liquids)
The reactants adsorb onto the catalyst and held onto the right position (correct orientation for a reaction to occur) - increase the surface area for possible collisions
No collisions occur, as reactants are held in place and their bonds are stretched and vulnerable to breakage
Then they desorb and go away as products
Higher surface area = more sites of adsorption
Catalysis
The process of employing a catlyst
Example of Homogenous Catlysis
Chlorine atoms acting as catalysts, breaking done ozone gas into oxygen gas - depleting the ozone layer
Why Heterogenous Catalysts Are Used For Industrial Purposes
More easily seperated from products of reaction - can be reused and recycled
Much easier to use
Able to be used at high temperatures
Many homogeneous catalysts are molecular compounds (e.g., transition metal complexes).
High temperatures can:
Break down the catalyst (decompose it chemically)
Change its oxidation state or structure, making it inactive
They are dissolved in solution, so thermal stability is often low.
Result: The catalyst cannot survive long at high temperatures → reaction may slow or stop.
2. Heterogeneous catalysts
Definition: Catalysts in a different phase than reactants (usually solid with gas or liquid reactants).
Why they can withstand high temperatures:
Typically metals or metal oxides → very thermally stable
Structure doesn’t decompose easily
Can maintain activity even at hundreds of °C
Summary Of How To Alter Reaction Rates
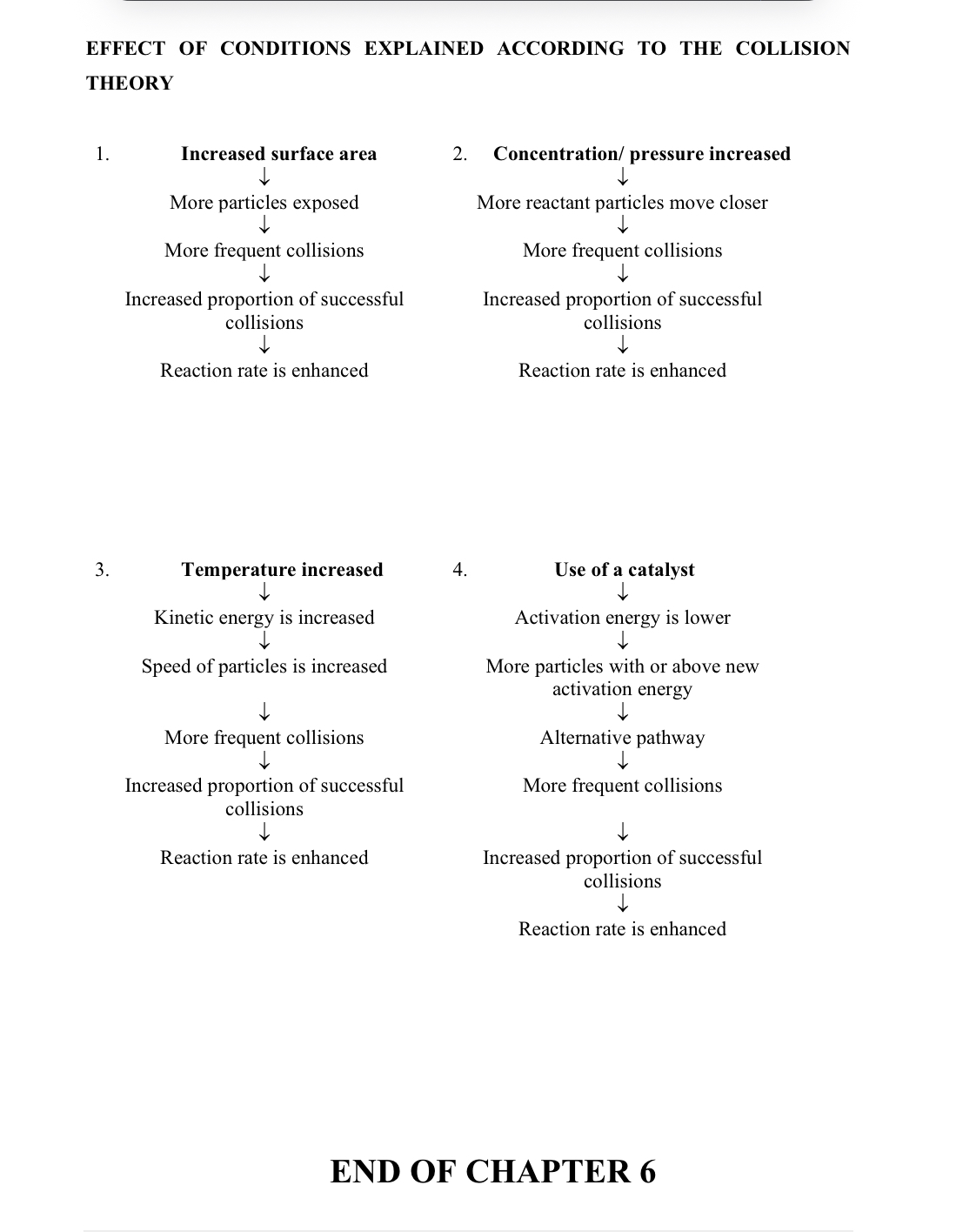
Why use a gas syringe
Used for accurately measuring collecting and dispensing volumes of mass
Why is cotton wool used on top of the container
It acts as a loose plug to allow gases to escape the vessel, whilst keeping the liquids within the vessel (preventing spraying or spilling)- especially during violent reactions
Surface Area Relationship
Larger the particle - surface area is smaller - smaller sites of reaction
Smaller the particle - Surface area is larger (e.g. powder)
SA = 1/particle size (inversely proportional - the larger the individual particle, the smaller it's surface area)