Fluids & Blood
1/228
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
229 Terms
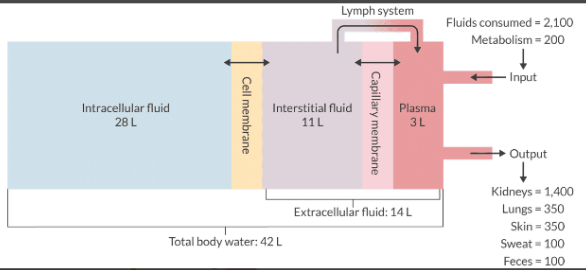
Describe the distribution of body water.
In the textbook 70-kg male, water represents 60% of the total body weight. This equals 42 L.
TBW can be divided into:
Intracellular volume = 40% of total body weight (28 L)
Extracellular volume = 20% of total body weight or (14 L)
ECV can be further divided into:
Interstitial fluid = 16% of total body weight (11 L)
Plasma fluid = 4% of total body weight or (3 L)
Remember: 60/40/20 (15/5) or (16/4) - either will work.
Which populations tend to have a greater percentage of TBW% by weight? Which have less?
Populations with higher TBW% by weight: Neonates
Populations with lower TBW% by weight: Females, obese, and elderly
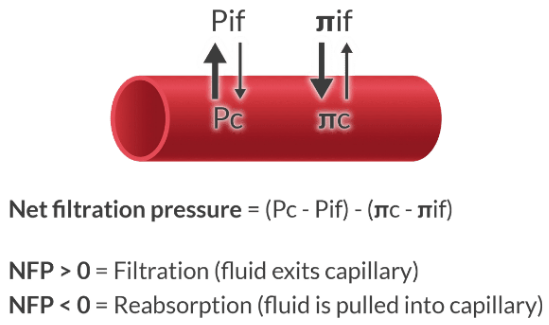
What are the 2 most important determinants of fluid transfer between the capillaries and interstitial space?
The plasma is in direct contact with the interstitial fluid by way of pores in the capillaries. The movement of fluid between the intravascular space and the interstitial space is determined by:
Starling forces
The glycocalyx
Describe the Starling forces in the context of capillary fluid transfer.
Forces that move fluid from the capillary to the interstitium:
Pc = Capillary hydrostatic pressure (pushes fluid out of capillary)
π if = Interstitial oncotic pressure (pulls fluid out of capillary)
Forces that move fluid from the interstitium into the capillary:
Pif = Interstitial hydrostatic pressure (pushes fluid into capillary)
π c = Capillary oncotic pressure (pulls fluid into capillary)
What is the glycocalyx, and what factors disrupt it?
The endothelial glycocalyx forms a protective layer on the interior wall of the blood vessel. It can be viewed as the gatekeeper that determines what can pass from the vessel into the interstitial space. It also contains anticoagulant properties.
Disruption of the glycocalyx contributes to capillary leak. Accumulation of fluid and debris in the interstitial space reduces tissue oxygenation. Conditions that impair the integrity of the glycocalyx include:
Sepsis
Ischemia
Diabetes mellitus
Major vascular surgery
What is lymph, and how does the lymphatic system work?
You can think of the lymphatic system as a fluid scavenger. It removes fluid, protein, bacteria, and debris that has entered the interstitium. It accomplishes this goal with a pumping mechanism that propels lymph through a vessel network lined with one-way valves. This creates a net negative pressure in the interstitial space.
Edema occurs when the lymphatic system is unable to do its job.
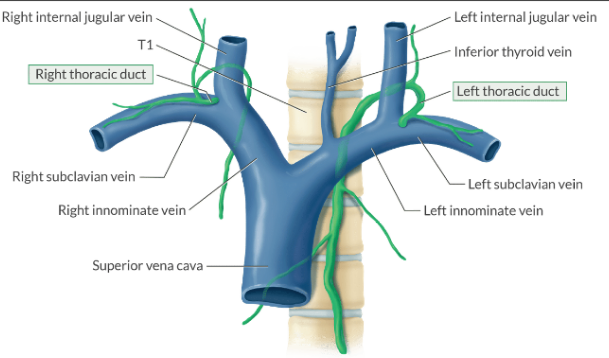
How is lymph returned to the systemic circulation?
Lymph is returned to the venous circulation by way of the thoracic duct at the juncture of the internal jugular and subclavian vein.
You can injure the thoracic duct during venous cannulation. Since the thoracic duct is larger on the left side, there is a greater risk of chylothorax (lymph in the chest) during left-sided IJ insertion.
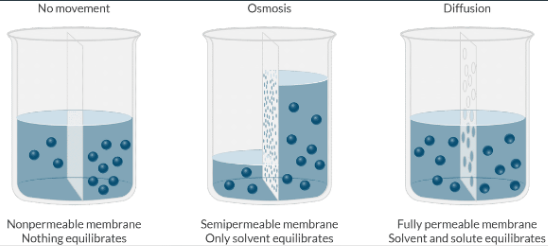
What is the difference between osmosis and diffusion?
Osmosis is the net movement of water across a semipermeable membrane, where the direction of water movement is driven by the difference in solute concentration on either side of the membrane (only the solvent moves).
Diffusion is the net movement of molecules from a region of high concentration to a region of low concentration (solvent AND solute move).
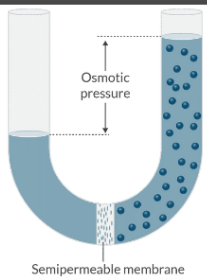
What is osmotic pressure, and what is its primary determinant?
Osmotic pressure is the pressure of a solution against a semipermeable membrane that prevents water from diffusing across that membrane.
Osmotic pressure is a function of the number of osmotically active particles in a solution.
It is NOT a function of their molecular weights.
What's the difference between osmolarity and osmolality?
Both are measures of concentration - the amount of solvent within a defined space.
Osmolarity measures the number of osmoles per liter solution.
Osmolality measures the number of osmoles per kilogram of solvent.
What is the reference value for plasma osmolarity, and what are the 3 most important contributors?
Plasma osmolarity is normally 280 - 290 mOsm/L.
The 3 most important determinants are: sodium, glucose, and BUN.
Plasma osmolarity = 2[Na+] + Glucose/18 + BUN/2.8
From this equation, you can see that sodium is the most important determinant of plasma osmolarity..
You should also notice that hyperglycemia or uremia can increase plasma osmolarity.
![<p>Plasma osmolarity is normally 280 - 290 mOsm/L.</p><p>The 3 most important determinants are: sodium, glucose, and BUN.</p><ul><li><p><em>Plasma osmolarity = 2[Na+] + Glucose/18 + BUN/2.8</em></p></li></ul><p>From this equation, you can see that sodium is the most important determinant of plasma osmolarity..</p><ul><li><p>You should also notice that hyperglycemia or uremia can increase plasma osmolarity.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/1208f365-2a7f-4f81-900e-0090377e82e8.png)
What is the difference between a hypotonic and hypertonic solution?
Tonicity compares the osmolarity of a solution relative to the osmolarity of the plasma. Remember that plasma is the reference point we use to make the comparison.
Since plasma is isotonic to cells, we can think about tonicity another way. We can use it to compare the tonicity of a solution to the tonicity of the cells.
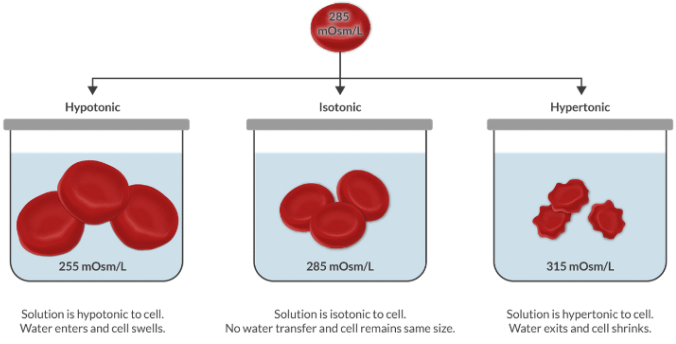
Think of all of the IV fluids you can. Which are hypo-, iso-, and hypertonic to plasma? Bonus points if you can list the osmolarity of each.
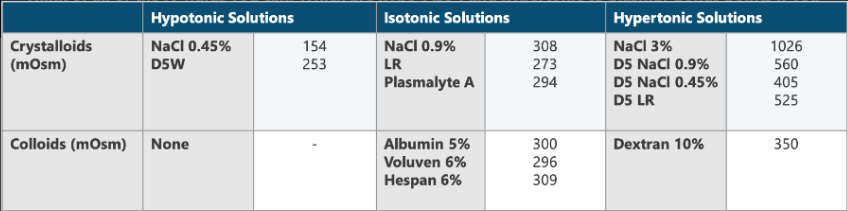
What is the relationship between the hypotonicity and hypertonicity of IV solutions and increased ICP?
Hypotonic solutions have a lower osmolarity than the plasma (or cells). These fluids are the same as giving free water, and this free water distributes throughout all of the body compartments.
This is why hypotonic solutions are poor expanders of intravascular volume and why you should never give a hypotonic solution to a patient with increased ICP. It will cause these cells to swell, increase their volume, and increase ICP!
Instead, hypertonic saline is useful for treating cerebral edema (it pulls water out of cells, causing them to shrink).
How does dextrose affect the tonicity of IV fluids?
You may be thinking that glucose in IVF (such as D5W) should be osmotically active. Well ... you're half right ...
You're right because the glucose contributes osmotically active molecules to the plasma.
The other side of the story is that this glucose is metabolized to carbon dioxide and water. What's left over? Water, and this water is, you guessed it ... hypotonic.
How do isotonic IV fluids distribute in the patient?
Isotonic solutions have an osmolarity that is very close to the plasma (or cells). These solutions expand the plasma volume and the ECV.
Crystalloids tend to remain in the intravascular space for ~ 30 min, before moving to the ECF.
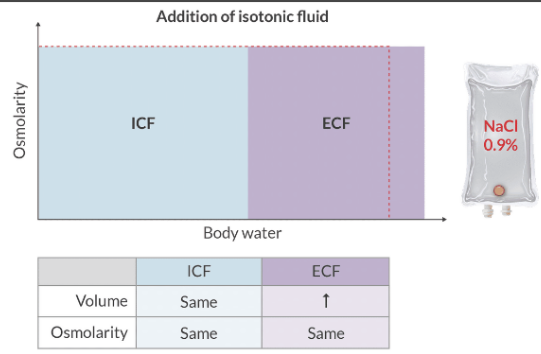
What complication can result when hypertonic saline is administered too quickly?
Central pontine myelinolysis
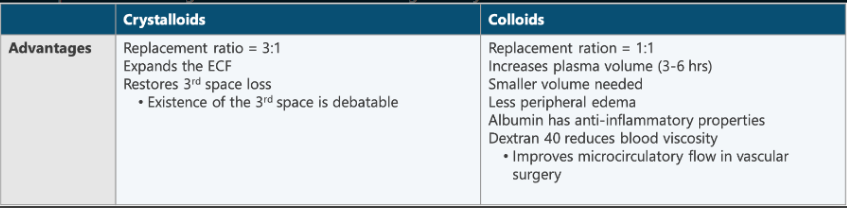
Compare the advantages of colloids to the advantages of crystalloids.
Crystalloids
Replacement ratio = 3:1
Expands ECF
Restores 3rd space loss (debatable)
Colloids
Replacement ratio = 1:1
Increases plasma volume (3~6 hrs)
Smaller volume needed
Less peripheral edema
Albumin has anti-inflammatory properties
Dextran 40 reduces blood viscosity
Improves microcirculatory flow in vascular surgery
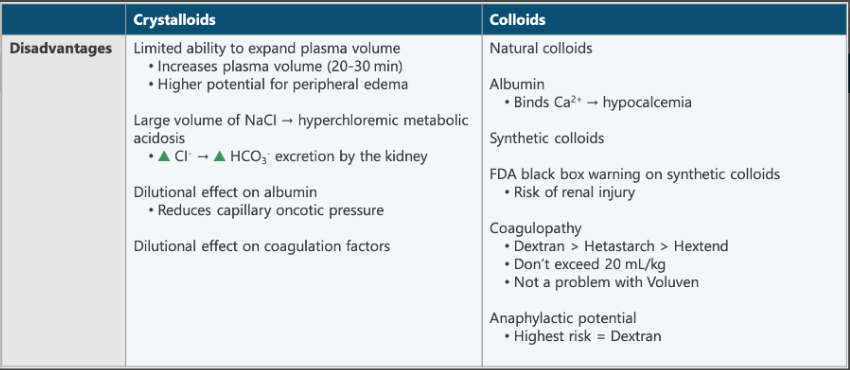
Compare the disadvantages of colloids to the disadvantages of crystalloids.
Crystalloids
Limited ability to expand plasma volume
NaCl - risk of hyperchloremic metabolic acidosis
Dilutional effect on albumin
Dilutional effect on coagulation factors
Colloids
Albumin
Binds to Ca+2 = hypocalcemia
Synthetic colloids (FDA black box warning) - risk of renal injury
Coagulopathy
Dextran > Hetastarch > Hextend
Don’t exceed 20 mL/kg
Anaphylactic potential
What is the black box warning on synthetic colloids?
Risk of renal injury
Which colloid has the highest incidence of coagulopathy?
Dextran > Hetastarch
What is the most common electrolyte disorder in clinical practice?
Hypokalemia
What is normal serum potassium?
3.5 - 5.5 mEq/L
What does potassium regulate?
Resting membrane potential in nervous tissue, skeletal muscle, and cardiac muscle.
How does hyperkalemia affect the EKG? (list the events in order of appearance)
Potassium = 5.5 - 6.5:
Peaked T waves
Potassium = 6.5 - 7.5:
P wave flattening
PR prolongation
Potassium 7.0 - 8.0:
QRS prolongation
Potassium ≥ 8.5:
QRS → sine wave → VF
How does hypokalemia affect the EKG?
PR interval = long
QT interval = long
T wave = flat
U wave
How do you treat hyperkalemia?
Cardiac membrane stabilization:
Calcium
Redistribution (shift K+ intracellularly):
Insulin + D50
Hyperventilation
Bicarbonate
Albuterol
Elimination:
Potassium wasting diuretics
Kayexalate
Dialysis
List 5 ways potassium is lost via the GI tract.
Vomiting/diarrhea
Nasogastric suctioning
Zollinger-Ellison syndrome
Jejunoileal bypass
Kayexalate
What is the maximum rate that K+ should be administered in a peripheral line?
10 mEq/hour
What is the maximum rate that K+ should be administered in a central line?
20 mEq/hour
When administering 3% saline for hyponatremia, the serum sodium concentration should be permitted to increase no faster than:
1 - 2 mEq/L/hr
Increase to quickly causes fluid shift from ICF to ECF = central pontine myelinolysis
Treating hypernatremia too quickly may cause:
Cerebral edema
What is the normal serum sodium value?
135 - 145 mEq/L
What is sodium the primary determinant of?
Serum osmolarity
Plays an important role in regulating the ECF volume through osmotic forces
For hypernatremia (Na > 145), presentations are based on serum osmolality:
350 - 375 = HA, agitation, confusion
376 - 400 = Weakness, tremors, ataxia
401 - 430 = Hyperreflexia, muscle twitching
> 431 = Seizures, coma, death
For hyponatremia (Na < 135), presentations are based on plasma sodium concentrations:
130 - 135 = No signs to mild signs
125 - 129 = N/V, malaise
115 - 124 = HA, lethargy, altered LOC
< 115 (or rapid onset) = Seizures, coma, cerebral edema, respiratory arrest
Surgery should be cancelled if the sodium concentration is less than:
130 mEg/L
What is the normal total plasma calcium and normal ionized plasma calcium?
Total = 8.5 - 10.5 mEq/L
Ionized = 4.65 - 5.28 mg/dL OR 2.2 - 2.6 mEq/L
What are the important functions of calcium?
Second messenger systems
Neurotransmitter release
Muscular contraction (skeletal, cardiac vascular, branchial, etc.)
Discuss the presentation of hypocalcemia.
Skeletal muscle cramps
Nerve irritability - paresthesia and tetany
Chvostek sign
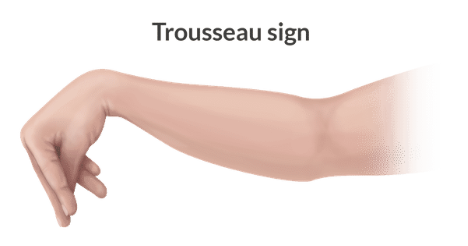
Trousseau sign
Laryngospasm
Mental status changes - seizures
Long QT interval
Discuss the presentation of hypercalcemia.
Nausea
Abdominal pain
Hypertension
Psychosis
Mental status changes → seizures
Short QT interval
What is Trousseau’s sign?
An upper extremity BP cuff is inflated above SBP for three minutes = muscle spasm of the hand and forearm (sign of hypocalcemia)
What is Chvostek’s sign?
Tapping on the angle of the jaw leads to facial contraction on the ipsilateral side (sign of hypocalcemia).

What hormone raises serum calcium?
Parathyroid hormone
Parathyroid glands release PTH
Osteoclasts release Ca+2 from bone
Ca+2 is reabsorbed by the kidneys
Ca+2 absorption in the small intestine increases via vitamin D synthesis
Ca+2 level in blood increases
What hormone lowers serum calcium?
Calcitonin
Thyroid gland releases calcitonin
Osteoclast activity is inhibited
Ca+2 reabsorption in the kidneys decreases
Ca+2 level in blood decreases
What is the most abundant electrolyte in the body?
Calcium (nearly all of it is stored in bone)
What is the treatment for hypercalcemia?
IV hydration (0.9% NaCl)
Loop diuretic (furosemide)
Loss of deep tendon reflexes is MOST likely a consequence of:
Hypermagnesemia
What is the normal plasma magnesium level?
1.7 - 2.5 mg/dL OR 1.5 - 2.1 mEq/L
Describe the presentation of hypermagnesemia.
Hypermagnesemia complication is usually caused by excessive adminstration (think OB and preeclampsia).
Loss deep tendon reflex = 5.8 - 10 mEq/L or 7 - 12 mg/dL
Respiratory depression = > 10 mEq/L or > 12 mg/dL
Cardiac arrest = > 10 mEq/L or > 12 mg/dL
What is the treatment for hypermagnesemia?
Calcium chloride or calcium gluconate
Ca antagonizes the effects of magnesium at the neuromuscular junction
How does hypermagnesemia affect neuromuscular blockade?
Hypermagnesemia potentiates neuromuscular blockade (Succ & nondepolarizers)
What are the clinical uses of magnesium?
Pre-eclamsia (4 g load IV over 10 - 15 minutues the 1 g/hr for 24 hours)
Mag crosses the placenta
Administration for > 48 hours increases the risk of neonatal respiratory depression, hypotension, and lethargy.
Opioid-sparing technique (NMDA receptor antagonism)
Acute bronchospasm
Cardia rhythm disturbances: symptomatic PVCs or torsades de points
What classic sign should be assessed in an OB patient receiving magnesium for preeclampsia?
Loss of deep tendon reflexes
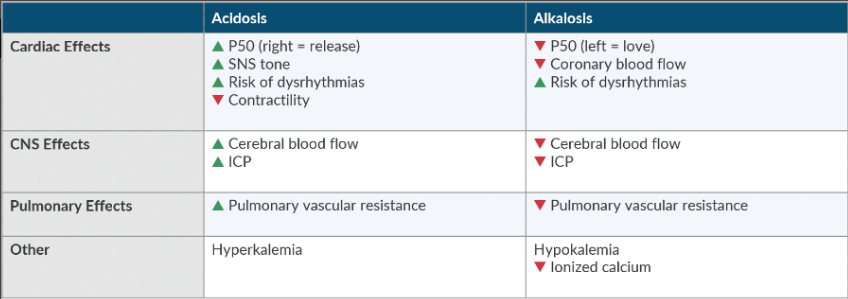
Compare and contrast the consequences of acidosis and alkalosis.
What is the anion gap, and what does it tell you?
The anion gap helps us determine the cause of the acidosis.
Anion ion gap = Major Cations - Major Anions or [Na+] - ([C[] + [HCO3]) = 8 -12 mEq/L is considered normal
Accumulation of acid (AG > 12) → gap acidosis
Loss of bicarbonate or ECF dilution → non-gap acidosis
What is the order of 5 questions to ask when evaluating pH?
Is the pH normal? (7.35 - 7.45)
Is the PaCO2 normal? (35 - 45 mmHg)
Is the HCO3- normal? (22 - 26 mEq/L)
Has compensation occurred?
If there’s metabolic acidosis, is the anion gap normal or increased?
List the possible causes of an anion gap acidosis.
Mnemonic: MUDPILES
Methanol
Uremia
Diabetic ketoacidosis
Paraldehyde
Isoniazid
Lactate (↓ DO2, sepsis, cyanide poisoning)
Ethanol, ethylene glycol
Salicylates (inhibits Krebs cycle)
List the possible causes of a non-gap acidosis.
Mnemonic: HARDUP
Hypoaldosteronism
Acetazolamide
Renal tubular acidosis
Diarrhea
Ureterosigmoid fistula
Pancreatic fistula
Large volume resuscitation with NaCI solutions can cause a non-gap metabolic acidosis with hyperchloremia (think trauma).
Causes of hypercarbia (respirtory acidosis) includes:
Increased CO2 production: Sepsis, thyroid storm, MH
Decreased CO2 elimination: Airway obstruction, COPD, opioid overdose
Rebrathing: Incompetent unidirectional valve, exhaused soda lima
When is mechanical ventilation indicated?
When pH is < 7.20
Causes of respiratory alkalosis include:
Iatrogenic (mechanical ventilation) (most common cause)
Pain
Pulmonary embolism
Hypoxia (high altitude, low FiO2, profound anemia)
Anxiety
Druges (progesterone, salicylates)
Reduced mechanical dead space with the same alveolar ventilation
For every 10 mmHg increases PaCO2 decreases pH by how much?
Acute Respiratory Acidosis: pH decreases by 0.08
Chronic Respiratory Acidosis: pH decreases by 0.03
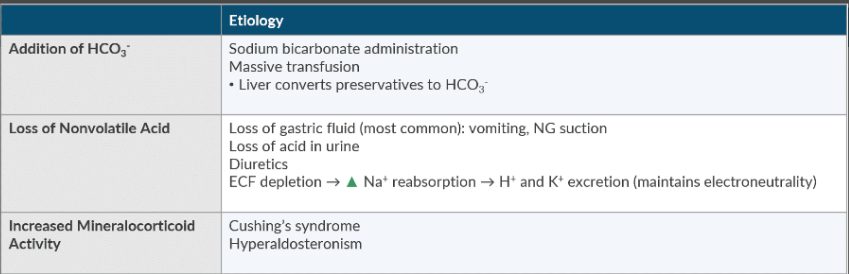
Discuss the etiology of metabolic alkalosis.
Massive blood transfusion
Vomiting
Diuretics
Cushing’s syndrome
Hyperaldosteronism
How do you treat metabolic alkalosis?
Treat the underlying cause
Acetazolamide
Spironolactone
Dialysis
What type of acidosis is caused by loss of bicarbonate?
Non-gap acidosis
What is the typical volume administered to assess the patient’s position on the Starling curve in goal-directed fluid therapy?
200 - 250 mL
What are 5 primary objectives to enhance postsurgical outcomes in an ERAS program?
Attenuate the physiologic changes that accompany surgical trauma.
Minimize the impact of fluid shifts.
Maximize the nutritional impact of healing.
Improve postoperative pain so patients can recover faster.
Improve patient education and compliance.
What are 5 intraoperative anesthetic actions used in the ERAS protocol that improve surgical outcome?
Short-acting drugs
Goal-directed fluid therapy
Maintain normothermia
PONV prophylaxis
Thoracic epidural when appropriate
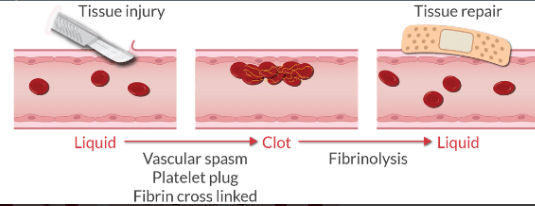
Under normal conditions, why does blood remain a liquid?
Blood exists as a viscous liquid. When there is no injury, blood remains a liquid because:
Coagulation proteins circulate in an inactive form.
The endothelium is smooth, and the glycocalyx repels clotting factors.
Undamaged endothelium does not express tissue factor or collagen. This prevents the activation of platelets and the coagulation cascade.
Activated factors are removed by brisk blood flow through the vessels as well as anticoagulants in circulation.
When there is a vascular injury, the blood plugs the damaged vessel by forming a clot (it solidifies). Over the next several days, the vessel repairs itself, and the clot is reabsorbed.
What are the 4 steps of hemostasis?
Vascular spasm
Formation of the platelet plug (primary hemostasis)
Coagulation and the formation of fibrin (secondary hemostasis)
Fibrinolysis when the clot is no longer needed
List 4 procoagulants:
Coagulation factors → Coagulation
Collagen → Tensile strength
wVF → Platelet adhesion
Fibronectin → Cell adhesion
List 5 anticoagulants:
Protein C → Degrades factor 5a and 8a
Protein S → Cofactor for protein C
Antithrombin → Inactivates 2a (thrombin) and factors 9a, 10a, 11a, 12a
Tissue pathway factor inhibitor → Inhibits tissue factor
Thrombomodulin → Regulates naturally occurring anticoagulants
List 3 fibrinolytics:
Plasminogen → Precusor to plasmin (breaks down fibrin)
tPA → Actives plasmin
Urokinase → Activates plasmin
List 2 antifibrinolytics:
Alpha-2 antiplasmin → Inhibits free plasmin in the blood
Plasminogen activator inhibitor → Binds to tPA and urokinase to accelerate clearance
List 5 different vasoactive mediators:
Vasoconstricion
Thromboxane A2 → Vascular smooth muscle constrictor
ADP → Vascular smooth muscle constrictor
Serotonin → Vascular smooth muscle constrictor
Vasodilation
Nitric oxide → Vascular smooth muscle relaxation
Prostacyclin → Vascular smooth muscle relaxation
Where are platelets formed? Where are they metabolized?
Platelets are:
Formed by megakaryocytes in the bone marrow.
Cleared by macrophages in the reticuloendothelial system and the spleen.
What is the normal value for platelets? What are the critical values?
Platelet count monitors the number of platelets, but not how well the platelets function. A normal count does not signify normal function.
Normal is 150,000 - 300,000 mm3
< 50,000 mm3 increases surgical bleeding risk
< 20,000 mm3 increases spontaneous bleeding risk
What is the lifespan of platelets?
8 - 12 days (1 - 2 weeks)
List the 9 structural components of platelets:
On External Membrane
Glycoproteins → Repelled by healthy vascular endothelium
Phospholipids → Substrate for prostaglandin synthesis (produce thromboxane A2 → activates platelets)
Inside Platelet
Actin and myosin → help form platelet plug
Thrombosthenin → Assists w/ platelet contractaction
ADP → Platelet activation and aggregation
Calcium → Multiple functions in coagulation cascade (factor 4)
Fibrin-stabilizing factor → Crosslinks fibrin (factor 13)
Serotonin → Activates nearby platelets
Growth factor → Helps repair damaged vessel walls
What are the 3 steps of platelet plug formation (primary hemostasis)?
After vascular injury occurs, platelets evolve into a platelet plug via a 3 step process:
Adhesion
Activation
Aggregation
The platelet plug is formed in ~5 minutes.
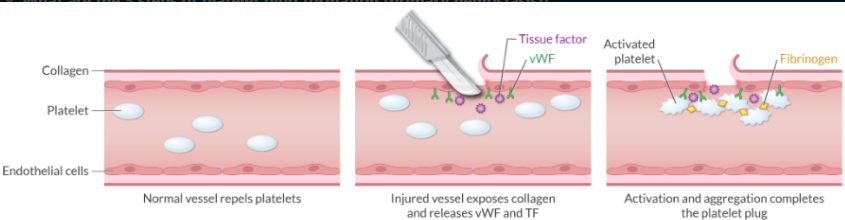
What substance is responsible for adhering the platelet to the damaged vessel?
Van Willebrand factor (Adhesion → step 1)
How does the injured blood vessel initially activate the platelet plug?
Endothelial injury exposes collagen. Exposed collagen at the site of vascular injury activates platelets.
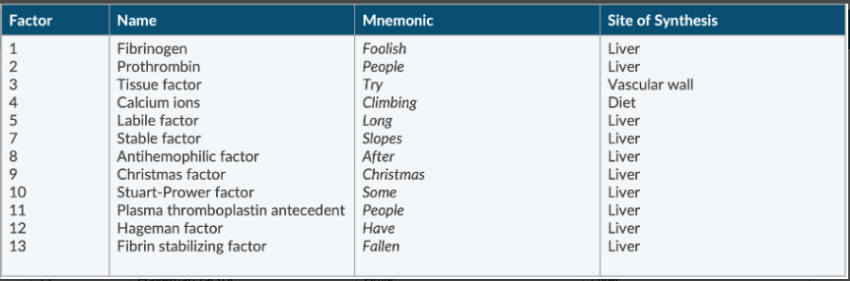
List the 12 coagulation factors.
There is no factor 6
Fibrinogen
Prothrombin
Tissue factor
Ca ions
Labile factor
Stable factor
Antihemophilic factor
Christmas factor
Stuart-Prower factor
Plasma thromboplastin antecedent
Hafeman factor
Fibrin stabilizing factor
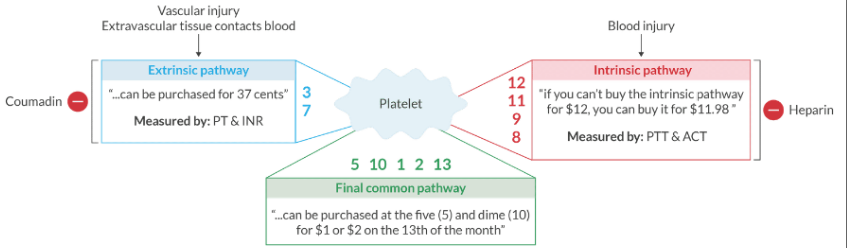
Regarding the extrinsic pathway: What activates it? What lab tests measure it? What drug inhibits it?
Vascular injury (tissue trauma liberates tissue factor from the subendothelium). Activated when coagulation is initiated outside of the intravascular space.
It's measured by the PT and INR.
It's inhibited by warfarin.
Factors 3 & 7
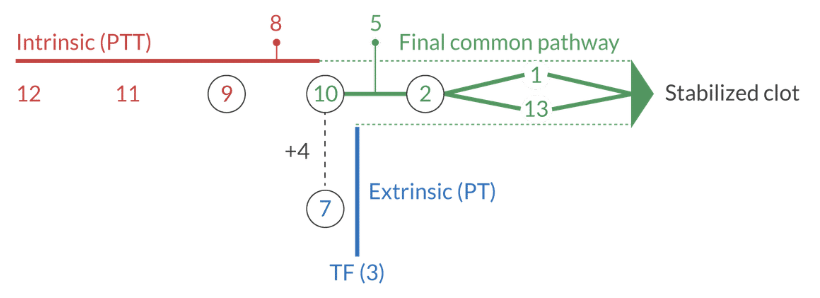
How fast can a clot form via the extrinsic pathway of the coagulation cascade?
~ 15 seconds
What is the first factor to be depleted in the patient with vitamin K deficiency?
Factor 7
Regarding the intrinsic pathway: What activates it? What lab tests measure it? What drug inhibits it?
Blood injury or exposure to collagen. Activated when coagulation is initiated inside the intravascular space
It's measured by the PTT and ACT.
It's inhibited by heparin.
Factors 8, 9, 11, & 12
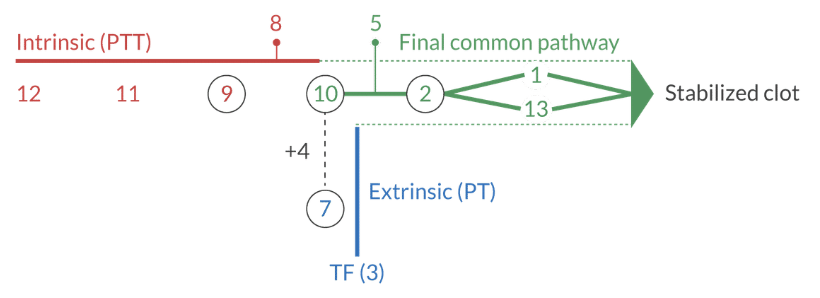
How long does it take to form a clot via the intrinsic pathway of the coagulation cascade?
Up to 6 minutes
A deficiency of what factor causes Hemophilia A?
Factor 8
What factors are in the extrinsic pathway, intrinsic pathway, and final common pathway?
Extrinsic pathway: 3, 7
" ... can be purchased for 37 cents"
Intrinsic pathway: 8, 9, 11, 12
"if you can't buy the intrinsic pathway for $12, you can buy it for $11.98.
Final common pathway: 1, 2, 5, 10, 13
" ... can be purchased at the 5 and dime (10) for 1 or 2 dollars on the 13th of the month"
Where does the final common pathway begin?
Begins where prothrombin activator (prothrombinase) changes prothrombin (2) to thrombin (2a).
What is the role of thrombin?
It converts fibrinogen to fibrinogen monomer.
What must be present to convert fibrinogen monomer to fibrin fibers?
Calcium (factor 4)
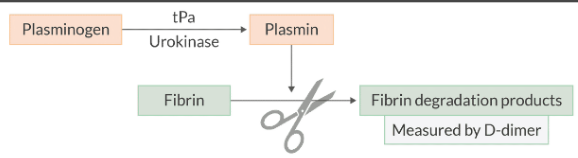
Describe the process of fibrinolysis.
Since the clot is only a temporary fix while the vessel repairs itself, the body must have a way to break down the clot after it is no longer needed. This process is called fibrinolysis.
Plasminogen is a proenzyme that is synthesized in the liver. It is incorporated into the clot as it's being formed, but it lays dormant until it is activated.
Plasmin is a proteolytic enzyme that degrades fibrin into fibrin degradation products.
What are 4 mechanisms that counterbalance clot formation?
Vasodilation and washout of ADP and TxA2
Antithrombin inactivating thrombin
Tissue factor pathway inhibitor neutralizes tissue factor
Release of protein C and S
What are 2 enzymes that convert plasminogen to plasmin?
tPA
Urokinase
Where is plasminogen synthesized?
Liver
It’s incorporated into the clot as it’s formed, but it lays dormant until a plasmin activates it (e.g., tPA, urokinase).
What 2 enzyme inhibitors turn-off the fibrinolytic process?
tPA inhibitor (tPAI)
Alpha-2 antiplasmin
How are plasmin activators used therapeutically?
They help dissolve thrombi to restore blood flow.