KJ - Thermodynamics
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
Define chemical equilibrium
The state (at constant temp or pressure) when the rate of conversion of reactions to products in the forward reaction is equal to the rate of conversion of products back to the reactants.
What does it mean when k>1 and k<1?
k>1 means there are more products than reactants
k<1 means there are more reactants than products
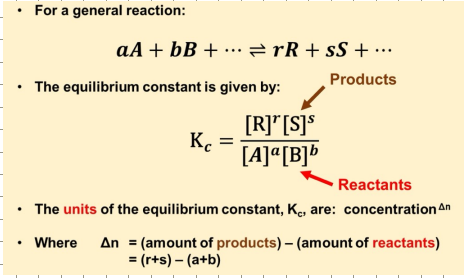
General equation for Kc of a reaction
General equation for Kp

General equation for KX

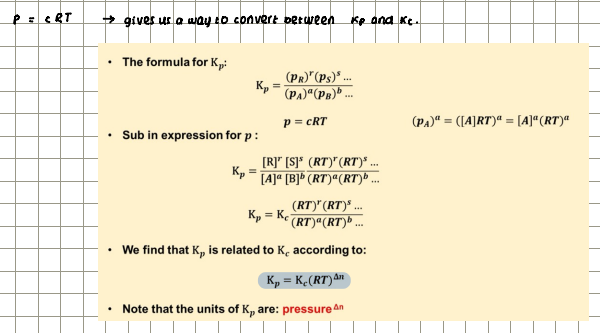
How is Kp related to Kc?
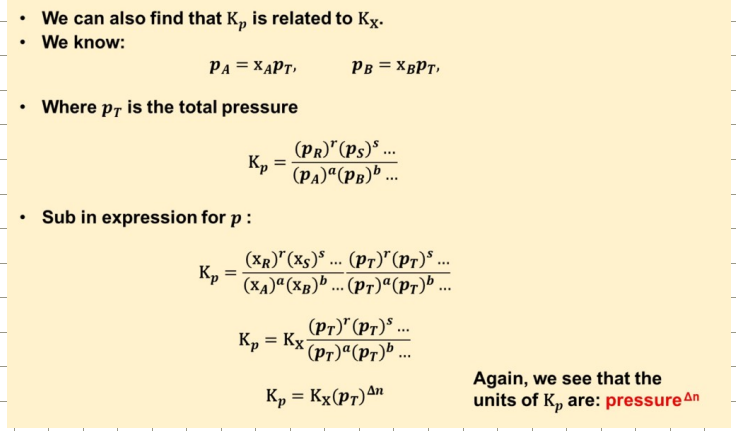
How is Kp related to Kx?
Calculate enthalpy
H = U + pV
Define state function
Commonly used in chemistry since many measurements are done at constant pressure.
Statements of the 2nd law of thermodynamics
The entropy of an isolated system can never decrease
Heat can never pass from a colder to a warmer body without some change occurring at the same time