organic scheme
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
ammonium salt → amide
dehydration heat strongly to liberate the water
carboxylic acid → ammonium salt
neutralisation ammonia NH3, ammonium carbonate (NH4)2CO2
carboxylic acid → acyl/ acid chloride
nucleophilic substitution
SOCl2 / PCl3 / PCl5
acid/ acyl chloride → N-substituted amide
Nucleophilic addition-elimination
Ammonia/ Amine dropwise
RTP
acyl/ acid chloride → carboxylic acid
Nucleophilic addition- elimination
H2O dropwise
RTP
acid / acyl chloride → ester
nucleophilic addition- elimination
alcohol dropwise
RTP
nitrile → carboxylic acid
nucleophilic addition (hydrolysis)
H+ catalyst & water
Reflux
nitrile → 1° amine
reduction
LiAlH4 in dry ether solvent
haloalkane → nitrile
nucleophilic substitution
potassium cyanide dissolved in ethanol (KCN)
warm
carboxylic acid → ester
esterification
H2SO4 / HCl catalyst & alcohol
reflux
ester → carboxylic acid
hydrolysis
H2SO4
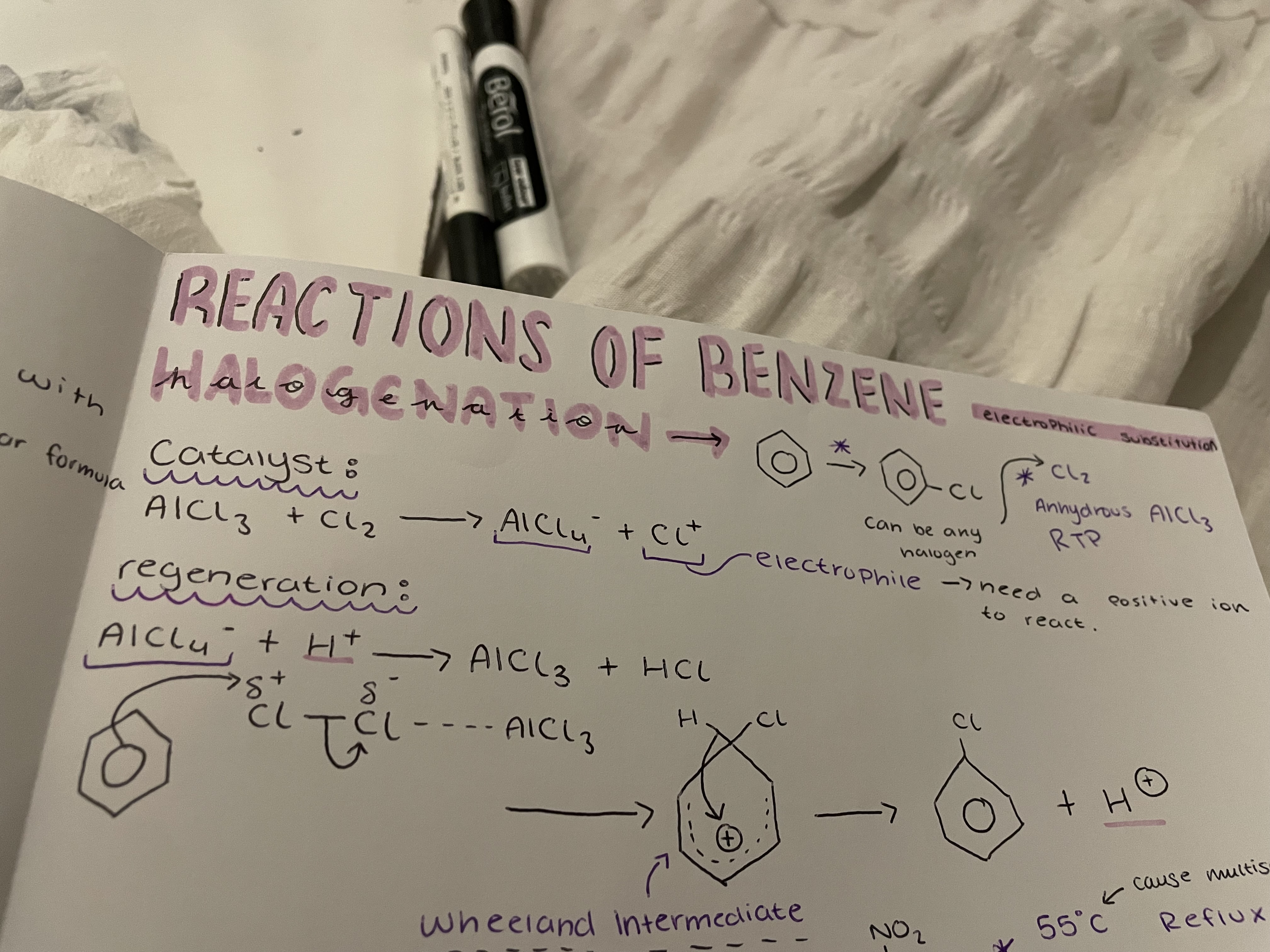
benzene → chlorobenzene (can be any halogen)
and catalyst regeneration
Halogenation reaction:
Cl2 and anhydrous AlCl3 RTP
Catalyst: AlCl3 + Cl2 → AlCl4^- + Cl^+
regeneration : AlCl4^- +H^+ → AlCl3 + HCl
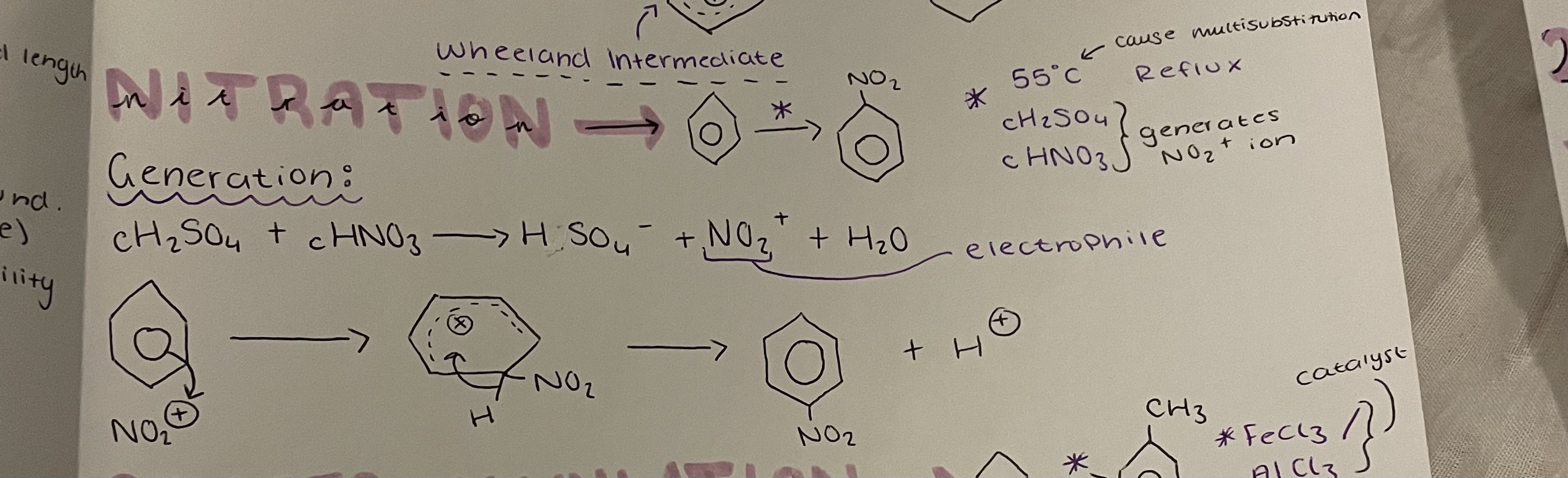
benzene→ nitrobenzene
Nitration
55°C, Reflux , cH2SO4 + cHNO3 generates NO2+ ion
Generation: cH2SO4 + cHNO3 → HSO4^- + NO2^+ + H2O
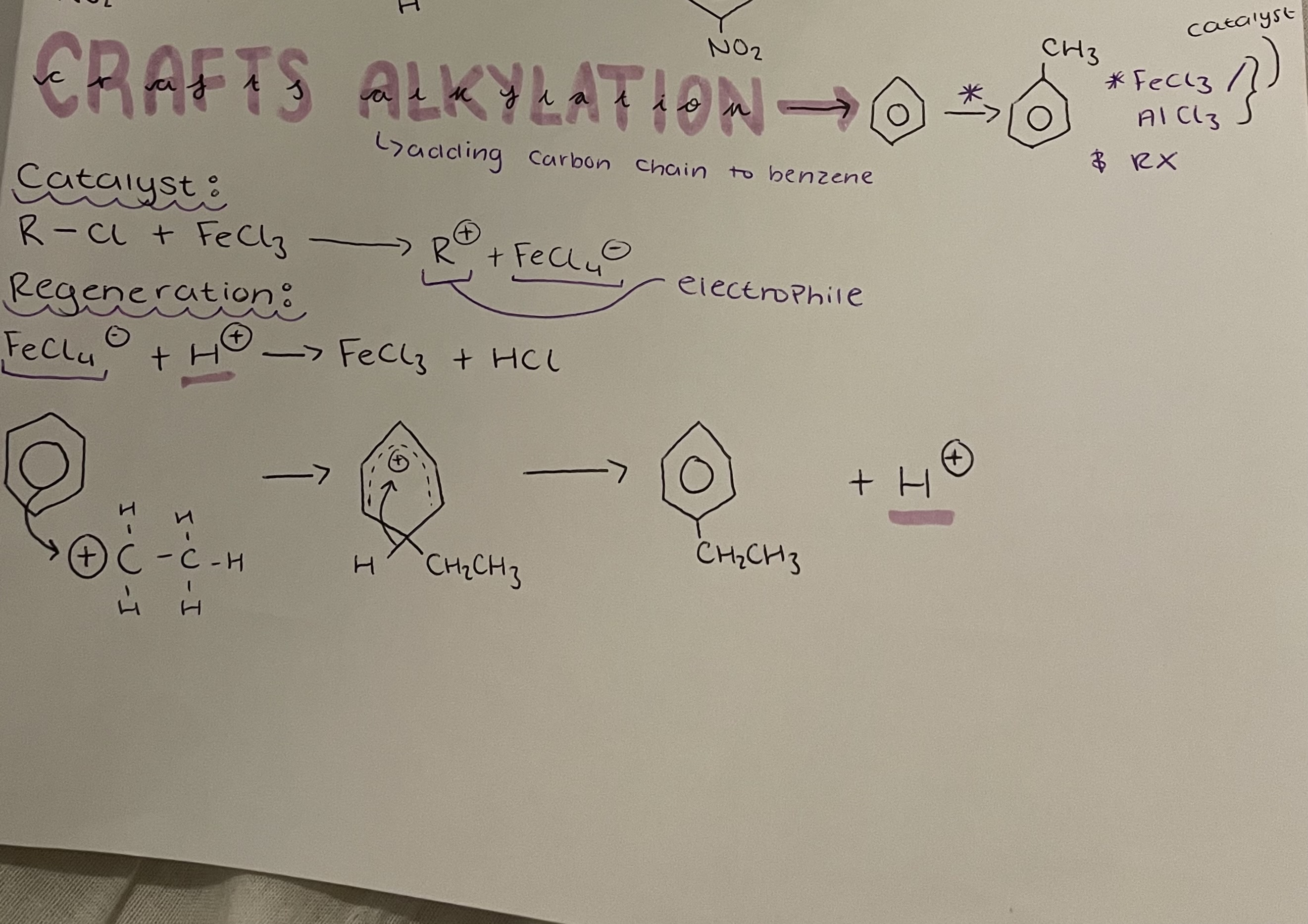
benzene → methyl benzene
Crafts Alkylation
FeCl3/ AlCl3 catalyst + RX
Catalyst: R-Cl + FeCl3 → R^+ + FeCl4^-
Regeneration: FeCl4^- + H^+ → FeCl3 + HCl
Do the alcohol and phenol flash cards
nitrile/ amide → Carboxylic acid
acid hydrolysis :
dH2SO4 & water under reflux - goes directly
Alkaline hydrolysis: dNaOH forms carboxylate salt which then add acid to protonate and make carboxylic acid
(nitrile needs water)
carboxylic acid → amide
2 step process:
add ammonia/ ammonium carbonate to make ammonium and carboxylate salt
heat strongly to dehydrate the salt and make amide
amide → nitrile
dehydration:
agent: phosphorus (V) oxide P4O10 & heat
forms water
haloalkane → nitrile
Nuc Sub
KCN (or NaCN) dissolved in ethanol
EXTENDS CARBON CHAIN
nitrile → amine
reduction
LiAlH4 in dry ether solvent
amide → carboxylate salt
alkaline hydrolysis
dilute H2SO4
carboxylate salt → carboxylic acid
neutralisation cH2SO4
carboxylic acid → carboxylate salt
neutralisation
add NaOH
2° alcohol → ketone
oxidation APD reflux orange → green
ketone → 2° alcohol
reduction LiAlH4/ NaBH4 RTP
ester → 1° alcohol
reduction LiAlH4 or NaBH4
ketone → hydroxynitrile
nucleophilic addition, HCN (hydrogen cyanide) RTP
CA → 1° alcohol
reduction LiALH4 in dry ether solvent RTP produces also water
1° alcohol → CA
APD full oxidation orange → green reflux
1° amine → 1° alcohol
electrophilic substitution
sodium nitrate NaNO2
cold nitrous acid HNO2 in situ
5°
haloalkane → 1° amine
nuc sub
excess alcoholic ammonia
reflux
haloalkane → nitrile
nuc sub
KCN
HCN dissolved in ethanol
Warm
haloalkane → 1° alcohol
nuc sub
NaOH reflux/ warm
1° alcohol → haloalkane
HX cH2SO4
haloalkane → alkane
elimination
NaOH distilled in ethanol
reflux
alkane → haloalkane
free radical sub
UV light
Cl2 (g)
alkene → haloalkane
electrophilic addition
alkane → alkene
Cracking
alkene → 1° alcohol
hydration/ addition
cH2SO4
300°C
60-70atm
1° alcohol → alkene
elimination / condensation
cH2SO4
170°C
aldehyde → 1° alcohol
reduction
NaBH4/ LiAlH4
RTP
1° alcohol → aldehyde
partial oxidation
APD
heat
distillation
orange → green
methyl benzene → salt
oxidation
alkaline potassium manganate
reflux
salt → benzoic acid
add dilute acid to protonate salt