Reactions of Alcohols
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
What are the 2 main methods of forming primary and secondary alcohols?
Substitution of halogen atom in a halogenoalkane by a hydroxyl group
Reduction of an aldehyde or ketone.
The substitution of halogen atom in a halogenoalkane by a hydroxyl group to form an alcohol is also known as what?
Alkaline hydrolysis
What type of reaction occurs when forming a alcohol from a halogenoalkane?
Nucleophilic substitution.
State the reagents and conditions needed to form an alcohol from a halogenoalkane.
Reagents: aqueous sodium hydroxide (NaOH).
Conditions: heated under reflux.
Describe how an alcohol can be formed from an aldehyde or ketone.
Using the reducing agent, sodium tetrahydriborate (NaBH4).
State the reactions of alcohol you need to know.
Alcohols with hydrogen halides
Alcohols with carboxylic acids
Alcohols react with hydrogen halides to form what?
Halogenoalkanes.
State the reagents and conditions needed to react an alcohol with hydrogen chloride.
Reagents: HCl gas.
Conditions: Anhydrous zinc chloride, ZnCl2
State the reagents and conditions needed to react an alcohol with hydrogen bromide.
Reagents: Potassium bromide (KBr).
Conditions: concentrated sulfuric acid, H2SO4
Alcohols react with carboxylic acids to form what?
Esters and water.
What is an ester?
A compound containing the -COO group bonded to alkyl or aryl groups.
Describe a key characteristic of esters.
Esters are sweet smelling liquids.
What reagents and conditions are used when reacting alcohols with carboxylic acids?
Reagents: Concentrated sulfuric acid catalyst
Conditions: Heated under reflux
State another method of forming esters apart from reacting alcohols with carboxylic acids.
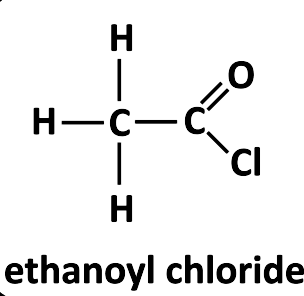
Reacting alcohols with an acid chloride e.g. ethanoyl chloride.
What is formed when alcohols are dehydrated?
Alkenes.
Concentrated sulfuric acid is needed to remove water.