(COMPLETE) LE2 (1) Red Blood Cells (RBC)
1/135
Earn XP
Description and Tags
Based on 2029 Trans
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
136 Terms
Blood is composed of?
1) 50% Plasma (ECF rich in proteins)
2) 45% Formed elements (RBC, WBC, Platelets)
T or F? Blood is a specialized connective tissue composed of:
8% in females
7% in males
False!
7% in Females
8% in Males
What is Hematocrit?
The fraction of the whole column occupied by RBC.
Calculate for Hematocrit?
Height of RBCs / Total Height of Column
T or F? Females generally have a higher amount of RBCs than Males.
False! Males> Females in terms of amount of RBC
What is the usual Hematocrit for:
Females
Males
Newborns
Females: 40%
Males: 45%
Newborns: 55%
what is the most abundant plasma protein?
Albumin
_ is the fluid portion taken from the blood after its allowed to clot.
Serum
Plasma
Serum
_ is the plasma that does not contain Clotting factors (I, II,V, VIII).
Serum
why are proteins in the blood important?
Proteins (e.g. Albumin) is a major contributor to oncotic pressure and helps in transportation of lipids and hormones.
What does oncotic pressure do?
Imagine a water balloon with some big marbles inside. The marbles are like special proteins in your blood.
Hydrostatic pressure is the water inside the balloon pushing on the balloon's walls, trying to get out.
Oncotic pressure is the marbles "sucking" the water back in. They act like magnets for water.
It's a tug-of-war! Hydrostatic pressure pushes fluid out, and oncotic pressure pulls it back in. This keeps the right amount of fluid in your blood vessels.
If there is High Oncotic pressure, water will tend to stay?
Inside the solution!
_ transports ions, hormones, lipids and has an immune function.
Fibrinogen
Regulatory proteins
Globulin
Albumin
Globulin
_ is involved in Clotting.
Fibrinogen
Regulatory proteins
Globulin
Albumin
Fibrinogen
_ are enzymes, clotting factors, hormones.
Fibrinogen
Regulatory proteins
Globulin
Albumin
Regulatory proteins
Where are plasma proteins mostly made?
Liver
What could cause Edema?
The liver doesnt produce enough Proteins in the blood (esp. Albumin).
This causes fluid to move into the interstitial space instead of the vessels.
Main function of RBCs?
Transport of Hemoglobin (Hgb)
Average RBC count for Males and Females?
Males: around 5 million per mL
Females: around 4.7 million per mL
Shape of RBCs
Biconcave DISCS
Consequence of RBCs lacking Nucleus and other organelles.
Cannot synthesize protein
Cannot perform oxidative metabolism
Has a limited ability to repair itself.
Primary method of ATP generation in RBCs?
Anaerobic Glycolysis
Average Diameter of an RBC?
7.8 µm in diameter
Reason for High Flexibility of RBCs
Their biconcave shape allows them to bend and deform without damaging the cell membrane.
Function of 2,3-diphosphoglycerate (2,3-DPG)
Acts on hemoglobin to reduce its affinity for oxygen, promoting oxygen release into tissues.
Substance Protecting RBCs from Oxidant Damage
Glutathione
Enzyme in RBCs for CO2 Transport
Carbonic anhydrase
Function of the Cl--HCO3- exchanger
Exchanges chloride ions for bicarbonate ions across the red blood cell membrane.
Location of RBC Synthesis (Mesoblastic Phase)
(embryonic development)
Yolk Sac
Primary Location of RBC Synthesis (Hepatic Phase)
(4-6 months of gestation)
Liver
Location of RBC Synthesis (Medullary Phase)
(from birth and till the person ages)
Bone Marrow
What does bone marrow 'cellularity' represent in the context of RBC synthesis?
A.
The total volume of bone marrow in the body.
B.
The speed at which red blood cells are produced.
C.
The percentage of red blood cells within the bone marrow.
D.
The amount of stem cells in the bone marrow needed for RBC synthesis.
The amount of stem cells in the bone marrow needed for RBC synthesis.
In which set of bones is RBC synthesis primarily concentrated during adulthood?
A.
Vertebra, sternum, and rib
B.
Tibia and femur
C.
All bones in the body
D.
Humerus and clavicle
Vertebra, sternum, and rib
What is the general trend of bone marrow cellularity as a person moves from young adulthood to old age?
A.
It fluctuates unpredictably.
B.
It steadily drops.
C.
It remains relatively constant.
D.
It increases significantly.
It steadily drops.
At approximately what age range does RBC synthesis in the tibia and femur significantly decline?
A.
65+ years old
B.
40-50 years old
C.
18-25 years old
D.
5-10 years old
18-25 years old
Which statement accurately describes the RBC production capability of a healthy elderly person?
A.
Their RBC production becomes concentrated in the tibia and femur again.
B.
They can no longer produce new red blood cells.
C.
Their bone marrow has the same cellularity as a young adult's.
D.
Their bone marrow is less cellular but can still produce an appropriate amount of RBCs.
Their bone marrow is less cellular but can still produce an appropriate amount of RBCs.
In the earliest stage of life, such as in a newborn, where does most RBC synthesis take place?
A.
Only in the vertebra and sternum
B.
Primarily in the liver and spleen
C.
Only in the tibia and femur
D.
In all bones
In all bones
The shift of primary RBC synthesis from long bones to axial bones is a direct consequence of what?
A.
Hormonal changes during puberty
B.
Changes in diet
C.
The level of physical activity
D.
Increasing age
Increasing age
Which of the following bones is NOT a primary site of RBC synthesis in adulthood?
A.
Rib
B.
Sternum
C.
Vertebra
D.
Femur
Femur
A lower bone marrow cellularity in an older adult implies they have...
A.
stronger but smaller bones.
B.
a more efficient method of RBC synthesis.
C.
fewer stem cells than a younger person.
fewer stem cells than a younger person.
where RBC synthesis sites become more localized over time.
concentration to axial bones with age.
Where do all blood cells derive from?
Pluripotent Hematopoietic Stem Cells found in the Bone Marrow.
After division, Pluripotent HSC can:
__ in the bone marrow
become ___
After division, Pluripotent HSC can:
Remain in the bone marrow
become Committed stem cells
What are the two possible lineages from Pluripotent HSC?
1) Myeloid - matures in the myeloid (bone marrow)
2) Lymphoid - matures in lymphoid organs
What cells come from the myeloid lineage?
Erythrocytes
Platelets
White Blood Cells (EXCEPT lymphocytes)
What cells come from the lymphoid lineage?
Lymphocytes! (Both T and B Lymphocytes)
What are Hematopoietic Cytokines?
e.g.
GM-CSF (granulocyte-macrophage colonystimulating factor)
G-CSF (granulocyte colonystimulating factor)
M-CSF (macrophage colonystimulating factor)
IL-3
IL-5
TPO (thrombopoietin)
EPO (Erythropoietin)
They act as signals that influence Pleuripotent HSC to differentiate into different Blood cells.
IL-3 affects which Blood cells?
Remember “3”
3= RBC, WBC & Platelets
IL-5 affects which Blood cells?
Remember “5” - 5th letter of the alphabet = E
Eosinophils!
Remember! All types of blood cells are produced via the ___ lineage, except the lymphocytes.
Myeloid lineage
As the RBC cell matures, (inc/dec)
_Hemoglobin
_ size of rbc cell
_ basophilic materials
_ nuclear size
As the RBC cell matures, (inc/dec)
Increase Hemoglobin
Decrease size of cell
Decrease basophilic materials
Decrease nuclear size Until finally Extruded!
List down the Stages of Erythropoiesis!
“Please Be Patient, Our Red Erythroblasts”
1) Pro-erythroblast
2) Basophil erythroblast
3) Polychromatophil erythroblast
4) Orthochromatic erythroblast
5) Reticulocyte
6) Erythrocyte
T or F? The Pro-erythroblast divides multiple times to produce MULTIPLE erythrocytes.
True!
What Stage of Erythropoiesis marks the start of Hgb synthesis where there is a large # of free ribosomes.
Basophilic Erythroblast
When the erythroblast turns blue to PINK, this stage is known as?
Polychromatophil erythroblast
When cells become filled with hgb.
Which erythroblast cell stage does the:
nucleus condense to a small size
final remnant is absorbed or extruded from the cell
Endoplasmic reticulum is absorbed
Polychromatic erythroblast
Which erythroblast cell stage does the ejected nucleus undergo phagocytosis by macrophages?
Orthochromatic erythroblast
Which erythroblast cell stage has a “fried egg” appearance?
Orthochromatic (“Omlette”) erythroblast
Which erythroblast cell stage contains remnants of organelles and exits the bone marrow to enter the capillaries?
Reticulocyte
How does the reticulocyte exit the bone marrow into the capillaries?
Via Diapedesis or squeezing through the pores of the capillary membrane.
a2y2 is also known as _ hemoglobin?
Fetal (Y) hemoglobin
a2b2 is also known as _ hemoglobin?
Adult (B) hemoglobin
How does RBC act as a blood buffer?
They contain large amounts of carbonic anhydrase which converts CO2 and water → Carbonic Acid → produce H+ ions = buffered.
What is the most important regulator of RBC production?
Tissue Oxygenation
What is stimulated when the body is experiencing Hypoxia?
Erythropoietin is released to stimulate RBC production.
Where is erythropoeitin primary stored and where else can it be produced.
Primary (90%): Kidney
Others: Liver
Hypoxia leads to an increased level of HIF-1 which serves as?
Transcription factor for hypoxia inducible genes in Erythropoietin (EPO) Genes.
What is the mechanism of action for HIF-1 during hypoxia?
HIF-1 + Hypoxia Response Element (found in erythropoeitin gene) → Increased erythropoeitin synthesis!
T or F? The maturation and rate of RBC production is heavy influenced by a person’s nutritional status.
True
What are the 2 vitamins important for RBC synthesis?
Vitamin 9 - Folic Acid
Vitamin 12
What are vitamins 9 and 12 used for?
For the formation of Thymidine Triphosphate = building block of DNA
Macrocytic/Megaloblastic anemia is when?
Bone marrow erythroblastic cells produce LARGER THAN NORMAL RBCs.
Macrocytic/Megalobastic anemia is caused by a defiency of which two vitamins?
Vitamin 9
Vitamin 12
What 2 proteins is RBC lipid bilayer membrane composed of?
Spectrin
Ankyrin
The hemoglobin is composed of:
__ Portion
__ heme groups (The iron carrying groups)
Globin Portion
4 heme groups
True or false? Hemoglobin is a tetrameric protein with 4 subunits.
true
T or F? Adult Hemoglobin’s subunit is composed of 2 Alpha and 2 Gamma chains
False! 1 alpha 2 beta
Remember!
Adult = a2b2
Child = a2y2
T or F? Heme group’s poryphyrin ring has an iron atom in the middle.
True
Which state (Relaxed State or Tensed State) of hemoglobin has a high affinity for oxygen?
Relaxed State
True or False? The T state of hemoglobin has a lower affinity for oxygen
T
when 100% saturated, 1g of Hemoglobin can combine with __mL of O2?
100% → 1g HgB = 1.34 mL O2
What does the oxygen-hemoglobin dissociation curve plot on its axes?
A.
Partial Pressure of Oxygen vs. Hemoglobin Saturation
B.
Time vs. Oxygen Consumption
C.
pH vs. Carbon Dioxide
D.
Temperature vs. Hemoglobin Concentration
Partial Pressure of Oxygen vs. Hemoglobin Saturation
The S-shape of the curve is a result of:
A.
The Bohr effect
B.
The flat portion buffering oxygen delivery
C.
Positive cooperative binding
D.
The steep portion facilitating oxygen unloading
Positive cooperative binding
A rightward shift of the oxygen-hemoglobin dissociation curve indicates:
A.
Increased hemoglobin affinity for oxygen and more difficult unloading in tissues
B.
Decreased hemoglobin affinity for oxygen and easier unloading in tissues
C.
Decreased hemoglobin affinity for oxygen and more difficult unloading in tissues
D.
Increased hemoglobin affinity for oxygen and easier unloading in tissues
Decreased hemoglobin affinity for oxygen and easier unloading in tissues
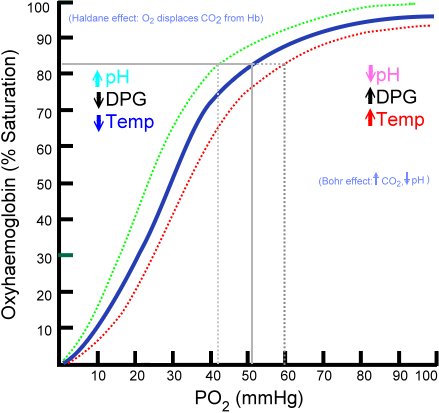
Which of the following would cause a leftward shift of the curve?
A.
Decreased pH
B.
Increased CO2
C.
Increased temperature
D.
Decreased 2,3-DPG
Decreased 2,3-DPG
The flat upper portion of the curve is most beneficial for oxygen transport at which location?
A.
Systemic capillaries
B.
Pulmonary capillaries (lungs)
C.
Kidneys
D.
Tissues with high metabolic demand
Pulmonary capillaries (lungs)
The flat portion of the curve ensures that even with a drop in oxygen partial pressure in the lungs, hemoglobin remains highly saturated, buffering oxygen loading.
A higher P50 value on the curve indicates a:
A.
Leftward shift of the curve and decreased oxygen affinity
B.
Rightward shift of the curve and decreased oxygen affinity
C.
Rightward shift of the curve and increased oxygen affinity
D.
Leftward shift of the curve and increased oxygen affinity
Rightward shift of the curve and decreased oxygen affinity
Fetal hemoglobin (HbF) has a different oxygen affinity than adult hemoglobin. How does the presence of HbF affect the curve?
A.
It causes a leftward shift, making it easier to unload oxygen to the fetus.
B.
It causes a leftward shift, making it easier to load oxygen from the mother's blood.
C.
It causes a rightward shift, making it harder to unload oxygen to the fetus.
D.
It causes a rightward shift, making it easier to unload oxygen to the fetus.
It causes a leftward shift, making it easier to load oxygen from the mother's blood.
When you exercise, your tissues produce more CO2, your temperature increases, and your pH decreases. How does the curve respond to these changes to facilitate oxygen delivery?
A.
The curve shifts to the left, increasing oxygen affinity, hindering oxygen delivery.
B.
The curve shifts to the left, decreasing oxygen affinity to offload more oxygen.
C.
The curve shifts to the right, decreasing oxygen affinity, allowing more oxygen to be released.
D.
The curve shifts to the right, increasing oxygen affinity to offload more oxygen.
The curve shifts to the right, decreasing oxygen affinity, allowing more oxygen to be released.
*What is the physiological significance of the steep portion of the curve?
A.
It acts as a buffer, ensuring oxygen saturation remains high despite pressure changes.
B.
It is not physiologically significant and is a byproduct of the binding process.
C.
It allows for the rapid unloading of large amounts of oxygen with a small drop in PO2 in the tissues.
D.
It allows hemoglobin to quickly become fully saturated with oxygen in the lungs.
It allows for the rapid unloading of large amounts of oxygen with a small drop in PO2 in the tissues.
CABET do the RIGHT thing
Inc. CO2
Inc. Acidity
Inc. 2,3 BPG
Inc Exercies
Inc Temp
If high P50 = _ affinity for O2?
Low affinity
In the body, Iron binds to
65% - Hemoglobin
15-30% - __
4% - Myoglobin
65% - Hemoglobin
15-30% - ferretin
4% - Myoglobin
A patient with hemochromatosis, a condition of iron overload, has a genetic mutation that results in abnormally low levels of hepcidin. Based on your understanding of iron regulation, what is the most likely consequence of this low hepcidin level?
A.
Decreased ferroportin activity, leading to less iron absorption.
B.
Increased ferroportin activity, leading to excessive iron absorption and release from storage.
C.
Increased ferritin synthesis, leading to more iron being stored in the enterocytes.
D.
Increased transferrin saturation, leading to less iron being delivered to the bone marrow.
Increased ferroportin activity, leading to excessive iron absorption and release from storage.
Remember! High Hepcidin = Low Ferroportin = Low Iron absorption
Hepcidin degrades ferroportin
Which of the following scenarios would most likely lead to a decrease in the body's hepcidin production?
A.
A bacterial infection causing systemic inflammation.
B.
Liver cirrhosis, hindering the liver's ability to metabolize iron.
C.
A diet rich in heme iron sources.
D.
An increase in erythropoiesis (red blood cell production) due to a blood-loss event.
An increase in erythropoiesis (red blood cell production) due to a blood-loss event.
A dietary supplement contains non-heme iron. Which substance from the list below, if consumed at the same time, would most significantly increase the absorption of this non-heme iron?
A.
Phytates (from grains or legumes)
B.
Tannins (from tea or coffee)
C.
Vitamin C (ascorbic acid)
D.
Calcium (from milk or supplements)
Vitamin C (ascorbic acid)
A medical professional needs to assess a patient's total body iron stores. Which of the following blood tests would be the most reliable indicator of the body's iron reserves?
A.
Serum Ferritin
B.
Total Iron-Binding Capacity (TIBC)
C.
Transferrin Saturation
D.
Serum Iron
Serum Ferritin
Remember! Ferritin = storage form of Iron
Which of the following correctly describes the process of non-heme iron absorption and its initial entry into the bloodstream?
A.
Ferrous (Fe2+) iron enters the enterocyte via ferroportin and is then transported to the blood by transferrin.
B.
Ferric (Fe3+) iron is transported into the enterocyte by DMT1 and is then exported to the blood via ferroportin as ferrous iron (Fe2+).
C.
Ferrous (Fe2+) iron is transported into the enterocyte via DMT1 and is then exported to the blood as ferric (Fe3+) iron via ferroportin.
D.
Ferrous (Fe2+) iron is transported into the enterocyte via DMT1 and then exported to the blood as ferrous iron (Fe2+) via ferroportin.
Ferrous (Fe2+) iron is transported into the enterocyte via DMT1 and then exported to the blood as ferrous iron (Fe2+) via ferroportin.
What is the primary role of transferrin in iron metabolism?
A.
To store excess iron within the liver.
B.
To convert dietary ferric iron (Fe3+) to ferrous iron (Fe2+) for absorption.
C.
To regulate the absorption of iron from the diet.
D.
To transport iron through the bloodstream to various tissues.
To transport iron through the bloodstream to various tissues.
. If a person has a deficiency in the protein hephaestin, what is the most likely consequence on iron metabolism?
A.
Reduced iron storage within enterocytes.
B.
Inability to absorb heme iron from the diet.
C.
Inability to export iron from enterocytes into the bloodstream.
D.
Excessive iron absorption due to a lack of regulation.
Inability to export iron from enterocytes into the bloodstream.
Hephaestin → oxidizes ferrous iron (Fe2+) to ferric iron (Fe3+) as it is exported from the enterocyte, allowing it to bind to transferrin. Without it, iron cannot be efficiently transported into the bloodstream.